Protein purification
RP-HPLC is an effective protein/peptide purification tool.
The target protein/polypeptide can be isolated from impurities by RP-HPLC, and the collected fragments can be used for further research, and analysis by orthogonal analysis techniques, and even as a therapeutic drug.
The goal of chromatographic optimization during protein/polypeptide analysis is to optimize resolution and retention time.
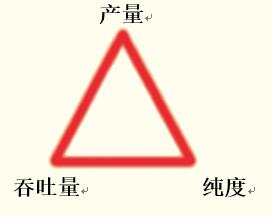
When preparative chromatography for protein/polypeptide separation, the development of chromatographic conditions is primarily optimized for three parameters (see Figure 45):
Yield is the purified target protein/polypeptide content obtained from each step of the chromatography. High yields increase the utility of the purification process and reduce costs.
Purity is the degree of removal of impurities from the target product. High purity helps to obtain better data from subsequent analyses or to obtain high purity products.
Flux is used to measure the mass of the material purified during the preparation cycle. High throughput illustrates the availability of more research or analytical materials, or more APIs, for the pharmaceutical industry at a given cost and time.
Since the purpose of preparative chromatography is different from the purpose of analytical chromatography, the optimization of chromatographic conditions is also different.
Figure 45. Optimization of separation conditions in the preparation and purification of proteins or peptides by seeking the optimal balance of yield, purity and flux.
Sample Loading <br> In analytical chromatography, a small sample is loaded onto the column to ensure that the amount of sample does not affect resolution.
If the sample volume is too high, the peak will widen and the resolution will decrease.
The amount of sample ("sample capacity") allowed to load into the column without peak broadening depends on the size of the column (appendix list shows column size and sample capacity).
When preparative chromatography purifies proteins/polypeptides, the sample capacity is often exceeded and the column is "overloaded", increasing yield and throughput (Figure 46).
When a certain resolution loss is allowed, the amount of sample loaded can be 10 to 50 times the sample capacity (appendix, maximum actual load).
In Fig. 46, although the peak is broadened due to the column overload, the peak shape is relatively good, indicating that the severe overload maintains the purity while increasing the yield, but the loss of the target protein/polypeptide is unavoidable.
The peaks in Figure 47 are also broadened due to sample overload.
Fragment Collection, Analysis, and Utilization <br> When the column is overloaded, the initial and ending peaks are usually discarded.
In Fig. 46, the middle region of the peak indicated by the red zone is collected. The peak and tail are removed.
This avoids the collection of impurity peaks with poor resolution, increases purity, but reduces yield.
In the preparative chromatography, several peaks of interest were collected for impurity analysis.
Figure 46. In the peptide purification example, preparative separation includes overload loading of the column to increase yield.
The resolution is reduced, and the peak and tail must be discarded in order to increase the purity, but the yield is slightly reduced. 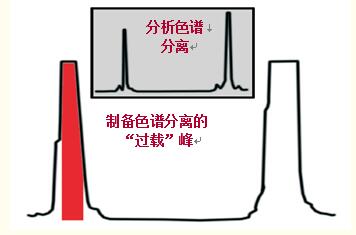
Based on the analysis results, fragments with little or no impurities were collected, and fragments with more impurities near the peak and the tail were discarded.
The choice of collecting and discarding fragments should take into account the balance between purity and yield.
For example, the 4.6 x 250 mm "analytical" column can be used to purify small amounts of peptide (up to about 200 micrograms) without loss of resolution.
However, in order to increase the yield and throughput, the same size column can purify up to 10 mg, but there will be some purity or yield loss.
Proper collection peak selection helps to achieve the best balance between purity and yield.
In preparative chromatography, although the focus is on sample quality, the sample volume can be large.
Although a sample loop and syringe can be used, more samples can be injected by "pumping" the sample onto the column.
Place the pump's suction tube in the sample container and load the sample through the elution pump to the column.
When the concentration of the organic solvent is low (usually, the sample is contained in the aqueous phase) and the protein/polypeptide of interest is eluted with an organic solvent gradient, a large amount of sample can be charged in this manner.
Particle Size of Adsorbent <br> The particle size of the adsorbent commonly used in analytical chromatography is 5 μm, and the particle size of the adsorbent commonly used in preparative chromatography is larger.
Especially when the sample loading exceeds the sample capacity (column overload), the column effect is less affected than the analytical chromatogram.
When the column is overloaded, the large particle size packed column has the same effect as the small particle size packed column to separate proteins and peptides.
Therefore, an adsorbent having a particle diameter of 10 μm or more is usually prepared for chromatography. The particle size distribution is also often wider.
Unlike the particle size distribution range of 0.5 μm or less, the preparative chromatography has a larger particle size range, for example, 10 to 15 μm.
Due to the back pressure and lower cost of preparative columns, larger particles are preferred.
Column inner diameter <br> Due to the low sample volume, small pore columns (inner diameter less than 2 mm) are rarely used in the purification process.
Small-scale laboratory purification uses a fine-pore column (about 2 mm inside diameter) and an analytical column (4.6 mm internal diameter).
The chromatographic conditions for this small scale separation are usually the same as those for analytical separation.
When a large amount of protein/polypeptide is required, a column of 10 mm and 22 mm inner diameter is used.
1 mg of protein or peptide can be purified using a 10 mm column and 5 mg can be purified using a 22 mm column.
When the column is overloaded, more protein/polypeptide can be purified. The 10mm column can purify up to 50mg, and the 22mm column can purify up to 200mg.
A large number of proteins or peptides are purified using large diameter columns of 50 mm, 100 mm or larger internal diameter.
It is known that up to 5 grams of protein/polypeptide can be purified on a 50 mm inner diameter column.
Column length <br> The preparation column is often relatively short compared to the analytical column.
This is because in preparative chromatography, the total volume of the column is more important than the column length, especially the separation of proteins.
Columns with an inner diameter of 60 cm and a column length of 12-15 cm ("cake" column) have been applied to large-scale purification of protein therapeutic drugs.
Because columns are often overloaded in preparative chromatography, and the benefits are far less important than yield, purity, and throughput, column sizes are optimized based on their utility rather than benefit.
Mobile phase composition <br> As with analytical chromatography, a small-scale purification of the commonly used acetonitrile-TFA system using a 10 to 22 mm id column.
Large-scale purification usually uses a solvent such as ethanol instead of acetonitrile, and acetic acid instead of TFA.
Although the use of these solvents as mobile phases reduces resolution, they are more suitable for large-scale use, and the resolution reduction is the same as the resolution loss inherent in column overload.
Protein denaturation <br> It is generally believed that reversed-phase chromatography denatures proteins, so the eluted proteins are not native proteins and may not be biologically active.
Although the operating conditions of reverse phase HPLC denature proteins, natural, biologically active proteins can be obtained after elution.
Organic solvents may weaken hydrophobic forces and cause loss of protein tertiary structure. The hydrophobic side of the adsorbent may also cause unfolding of the protein.
However, protein unfolding is generally slower than chromatographic separation time, and the protein only undergoes slight denaturation during reverse phase chromatography separation.
Due to the action of the disulfide bond, the protein remains spherical and only partially unfolded, so the protein eluted from the reverse phase column can usually be restored by restoring its natural structure by treatment in the appropriate refolding buffer. Natural state.
There are many examples showing that proteins purified by reverse phase HPLC still maintain the natural tertiary structure and biological activity.
Reversed phase purification of trypsin, where activity is retained and subsequently used for trypsin digestion of proteins.
Recombinant human erythropoietin is a successfully commercialized protein therapeutic that uses reversed-phase high performance liquid chromatography to separate protein drugs from their cell culture expression systems.
Another commercial protein therapeutic, granulocyte stimulating factor, was purified by reverse phase HPLC.
In addition, recombinant human insulin was purified by reverse phase HPLC to maintain the active structure.
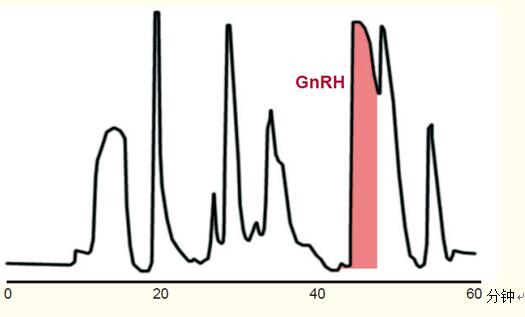
Purification Example <br> Figure 47 shows the purification process of a synthetic peptide, a gonadotropin releasing hormone (GnRH) antagonist. The purification process is carried out in the following steps:
Elution conditions were constructed on a 4.6 x 250 mm analytical column.
A 1.2 gram synthetic peptide mixture was loaded on a 50 x 300 mm column and eluted based on the conditions of the first step construction (Figure 47).
Fragments of each eluted peak of the GnRH antagonist were collected and analyzed by analytical analysis to achieve maximum yield and purity.
The lysate was again subjected to chromatographic analysis on a reversed-phase column using acetonitrile and TFA as an eluent to complete desalting of the collected fraction.
Collect fragments to achieve maximum yield and purity.
In this chromatographic purification step, 128 mg of purified peptide was collected from a 1.2 gm reaction mixture.
The purification process uses the same organic solvent as the analytical chromatographic separation, acetonitrile, but replaces TFA with triethylamine phosphate.
Due to the column overload, the elution peak is wider and the resolution is not as good as the analytical chromatogram. However, the peaks are still relatively tight, and it is easy to collect an eluate containing the polypeptide of interest and GnRH.
Figure 47. Purification of 128 mg of synthetic peptide, gonadotropin releasing hormone.
The column is heavily overloaded, resulting in a very wide peak.
condition
Column: C18 wide-bore column, 15~20μm particle size, 50 x 300 mm.
Mobile phase: gradient elution with acetonitrile and aqueous triethylamine phosphate.
Sample: Gonadotropin releasing hormone
This 50m distance measuring meter is made of two parts: the laser distance outside unit and the sole part laser measure module. Great distance range is from 3cm to 50 meters with ±1.5mm high accuracy. Digital distance measuring device is quick and reliable measure with one hand pushing the button. It is designed for hand helding, very convenient to put in your jacket pocket or trouser pocket.
1>. Easy to Use. It is very easy to learn how to use a Laser Distance Meter, it can measure distance with pressing a single button.
2>. Safety. Using a laser distance meter to measure is much safer than using a measure tape, for users do not need to climb up and down.
50M Laser Distance Measurer,Mini Laser Measurer, Laser Measure 50M,50M Laser Distance Meter
Chengdu JRT Meter Technology Co., Ltd , http://www.accuracysensor.com