Progress in the development of acute myeloid leukemia (AML)
February 12, 2018 Source: Sina medicine Author: April Chen
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];In the past four decades, standard treatment for acute myeloid leukemia (AML) has been stagnant, and the 5-year survival rate for patients over 60 years of age is still less than 10%. After years of bottlenecks in AML medication, 2017 Finally, there has been a lot of progress.
2017.06
Agios ivosidenib was combined with Vidaza (azacitidine) for the initiation of Phase III trials in patients with R/R IDH1 + AML;
Due to the death of the patient, Seattle Genetics terminated the vadastuximab talirine for a phase III trial of AML first-line treatment called CASCADE.
2017.07
Pfizer's Mylotarg (gemtuzumab ozogamicin) is supported by the FDA Oncology Expert Advisory Committee (ODAC);
AbbVie/Roche's Venclexta (venetoclax) in combination with low-dose cytarabine for untreated elderly patients with AML received FDA-approved breakthrough therapy
2017.08
FDA approves Ighifa (enasidenib) from Agios/Celgene for IDH2 mutant AML;
FDA approves Jazz's Vyxeos (liposomal daunorubicin and cytarabine) for high-risk AML elderly patients;
Arog's crenolanib in combination with Rydapt (midostaurin) for the initiation of a key phase III clinical trial of the head-to-head name PANTHER in FLT3 + AML patients
2017.09
FDA approves Mylotarg (gemtuzumab ozogamicin) for the treatment of newly diagnosed CD33 + AML patients;
The European Union has approved Novartis's Rydapt (midostaurin) for the treatment of newly diagnosed FLT3 + AML patients;
2017.10
The FDA grants gilteritinib a quick review of Astellas;
2017.11
Jazz's Vyxeos (daunorubicin + cytarabine) compound liposome injection) entered the EU rapid review stage;
2017.12
Agios ivosidenib is listed for R/R IDH1 mutation AML based on the results of Phase I trials;
Arog's crenolanib is used for R/R AML patients to obtain FDA fast track;
2018.01
Celgene acquired JUNO for $9 billion, and the latter's JTCR016 is being used for high-risk relapsing AML Phase I/II clinical trials.
1. New AML drugs approved in 2017

2, Rydapt preempts 30% of the FLT3 mutant population
The FDA approved Rydapt (midostaurin, PKC412) two indications in April 2017: First, in combination with glucosinolate and daunorubicin for patients with newly diagnosed FLT3 mutations over the age of 25, compared with standard chemotherapy alone. Reduce the risk of death by 23%; Second, systemic mastocytosis, is the only drug for this rare hypertrophic hyperplasia of blood disease, the total response rate of 21%.
3, Mylotarg has re-listed after twists and turns
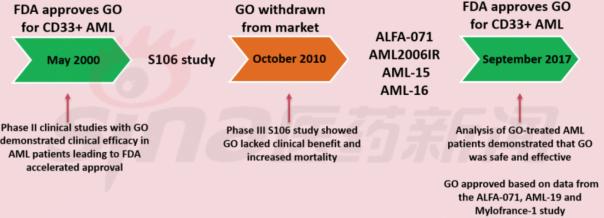
Mylotarg, the first FDA-approved ADC, was actively delisted in 2010 due to an increase in treatment-related mortality (5% vs 1%) in the 6 mg/m2 combination with the cytarabine group and the cytarabine control group. After a series of trials, the dose was reduced to a safer and more effective 3 mg/m2, which was finally approved by the FDA in September 2017 for use in combination with standard chemotherapy for newly diagnosed CD33AML and monotherapy for refractory relapse. In patients with CD33+AML, CD33 is expressed in 80-90% of AML patients, and it is also found to be associated with poor prognosis of FLT3-ITD. There are also studies on the combination of Mylotarg and chemotherapeutic drugs for FLT3-ITD mutant AML, which is expected to expand. Indications. Another progress in the phase III vadastuximab talirine was terminated due to the death of the patient, giving Mylotarg more market opportunities.
4. New base and Agios win in Enasidenib
Enasidenib is the first small molecule oral isocitrate dehydrogenase 2 (IDH2) inhibitor developed by Agios in collaboration with Shinki. Isocitrate dehydrogenases 1 and 2 (IDH1 and 2) can convert isocitrate to alpha-ketoglutarate, but in different positions in the cell, IDH1 is in the cytoplasm, IDH2 is in the mitochondria, both mutations Both increase the 2-hydroxyglutaric acid instead of isocitrate and affect the cell pressure in chemotherapy. Both IDH1 and 2 antibodies inhibit enzyme activity, limit cell pressure, and differentially regulate immature leukocytes in AML to mature cells by epigenetic regulation.
In fact, IDH1 mutations are not only in acute myeloid leukemia, but also in diffuse glioma, chondrosarcoma and intrahepatic cholangiocarcinoma. Another non-selective IDH1/2 mutation inhibitor, AG-881, which works with Shinki, has good blood-brain barrier permeability and is currently used primarily for central nervous system-related cancer research.
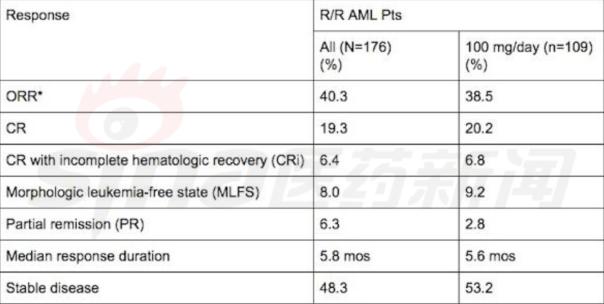
At the 2015 Annual Meeting of the American Society of Hematology (ASH2015), Shinki announced the early results of the one-arm, I/II AG221-C-001 study of enasidenib in patients with relapsed or refractory AML. Enasidenib's approval has been accelerated in two areas, one based on early 1/2 clinical trial data, and the second is awarded a rapid approval channel, with approval time reduced from 10 months to 6 months.
The overall IDH2 mutation is 8% to 19% in all AML patients, and there are approximately 1,200 to 1,500 patients in the United States. The patient is essentially drug-free before enasidenib is approved. This is the first drug that New Base has partnered with Agios. Agios is primarily a cancer metabolism platform, and the new base provides development and commercial resources. Enasidenib and the acquisition of JUNO, Shinki hopes to pass through all kinds of blood cancer.
Enasidenib clinical results
Agios product line
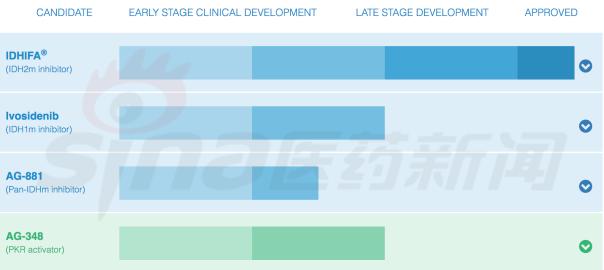
At present, other companies do not have a target product in the late pipeline, so Agios and Celgene will not face competition in the short term. The next Agios project to be listed at the end of 2018 is Ivosidenib.
5. Standard chemotherapy has finally made new progress
There is no change in the standard chemotherapy prescription. Vyxeos is based on JAZZ Pharmaceutical's acquisition of Celator Pharmaceutical's technology platform, developed glucosinolate and daunorubicin, a classic 7+3 chemotherapeutic 5 to 1 formula liposome injection for new The confirmed treatment-related AML (t-AML) and associated myelodysplastic-associated AML (AML-MRC) phase III trials had a median overall survival of 9.56 months, a significant increase from the control group of 5.95 months ( HR = 0.69; P = 0.005). The cytarabine-based chemotherapy market will split a portion into safer and more effective Vyxeos.
Urinalysis test strips refer to test strips that test for bilirubin, urobilinogen, ketone bodies, ascorbic acid, glucose, protein (albumin), blood cells, PH, etc. in urine.
Detection principle
1. pH: The pH value in the range of 5-9 is measured by the pH indicator, and the pH value of the fresh urine of a normal person is between 5-7.
2. Nitrite: The reaction is based on the reduction of nitrate to nitrite by Gram-positive bacteria in the urine. The nitrite reacts with p-aminobenzenesulfonic acid to form diazonium compounds, which are then combined with N-(1-naphthalene) )-3 aminopropanesulfonate combined with a pink color.
3. Glucose: According to the reaction principle of glucose oxidase, glucose oxidase specifically oxidizes glucose to generate glucuronic acid and hydrogen peroxide. Under the action of hydrogen peroxide, hydrogen peroxide oxidizes the indicator and turns color. .
Classification
Urinalysis test strips are divided into visual series and machine series. The visual inspection series is divided into several models according to different inspection items; the machine inspection series is divided into several models according to different applicable instruments.
1. Classification by measurement method
1) Visual inspection series
When observing the result, compare the color with the standard color code within the time specified on the color code, judge and read the result.
2) Machine test series.
For instrument operation, refer to the instruction manual of the Urine Analyzer used.
2. According to the number of measurement items
There are single-item, 2-item, 4-item and multiple test strips. Currently, 10-item or 11-item multiple test strips are most commonly used in hospitals.
3. Classification by structure
Urinalysis test strips with single-layer membrane structure and multi-layer membrane structure.
Urine Reagent Strips,Urine Test Strip,Urine Sugar Strip Test,Visual Urine Analysis Strips
Jilin Sinoscience Technology Co. LTD , https://www.jilinsinoscience.com