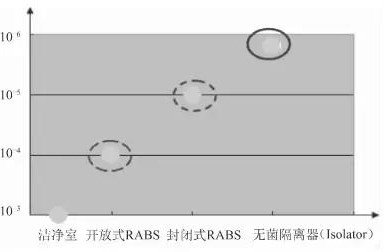
From the mid-1980s, sterility testing isolator (also known as sterility testing isolator) was first developed in Europe. This equipment can provide the most reliable environment for microbiological testing, providing a sterile environment for sterility testing. It can prevent microbial contamination of the sample to be tested, avoid contamination of test and auxiliary equipment, and avoid false positives. It has improved the accuracy of sterility test results and is now widely used in the global pharmaceutical industry. With the continuous development and improvement of Chinese Pharmacopoeia and GMP requirements, aseptic detection isolators are increasingly receiving attention from domestic pharmaceutical companies.
Vaporized Hydrogen Peroxide (VHP), also known as Hydrogen Peroxide Vapor (HPV), is an advanced high-level green disinfection technology. Launched in the 1980s, it was first applied to the sterilization of sterile isolators in the pharmaceutical industry. In recent years, it has been widely used in the sterilization treatment of high-level biosafety laboratories, the disinfection of confined spaces in the field of infectious diseases, the elimination of pollution of hospital super-resistant bacteria, and even the disinfection of closed aircraft cabins.
Sterilization of sterility test isolators is currently carried out using hydrogen peroxide vapor sterilant. A hydrogen peroxide generator is integrated inside the isolator, and the high-concentration hydrogen peroxide solution can be converted into a gaseous state by a generator, uniformly distributed in the isolator chamber, and sterilized under a certain concentration and time, and After the sterilization is completed, the residual hydrogen peroxide vapor in the cabin is decomposed and decomposed by a ventilation system with a high-efficiency filter to achieve the final sterilization effect. After the sterility inspection isolators are completed, the microbial load on the internal environment of the tank shall meet the requirements of Class A cleanliness in the GMP regulations, and the sterilization process shall not affect the microorganisms in the articles and the test samples. ( Aseptic effect of sterile isolator - Luo Huiyan, Yan Dongzhen, Liu Jiayuan, Liao Xinyu, Cao Yi - "China Equipment Engineering" 2017.03 (below )
The aseptic isolator completely separates the production space from the surrounding environment with a fully enclosed system, and is equipped with a corresponding air handling unit (including high efficiency filter) and a hydrogen peroxide sterilizing device to automatically sterilize the environment in the isolator. Work such as air purification treatment has the highest safety. ( Advantages of Sterile Isolators and Their Development Trends - Ma Wei Sun Shilei - "China Pharmaceutical Equipment" · July 2013 · 7th Series )
Bioquell Qube, a design designed to perform sterility testing in a sterile environment to ensure the elimination of false positives. Combined with leading sterility testing systems, good technology and a solid manufacturing process, the Bioquell Qube Aseptic Test System effectively reduces risk during operation. It is fully GMP compliant, operating parameters and reagents are traceable, and each batch of data can be exported. It optimizes the design of the storage rack, efficient processing tools and samples, user-friendly operation, and fully integrates the market-leading sterilized test system and particle monitoring functions to make the process confident and manageable.
Characteristics
• Rapidly utilize the best practices and point-contact principles to ensure fast processes meet user requirements
• Adaptive Bioquell QUBE modular design allows users to design according to their process needs
• Fine design of reliability bio-indicators (BIs) and chemical indicators (CIs) ensures rapid system verification and minimizes downtime
• Effectiveness One module is biologically sterilized while other modules are operational, meaning that operating time has been maximized
• Integrated pre-engineered test pumps and monitoring instruments mean less time and cost to integrate
• Production samples are quickly transferred via an optional material transfer module
Bioquell QUBE is a modular aseptic operating platform that provides ISO 5/A environment for rapid sterilization of samples and material transfers for 6-log biological purification. The sterilization cycle is as low as 20 minutes, and both chambers can be tested while the sample is being sterilized for a dynamic and efficient workflow. Each module of Bioquell QUBE can be customized in many different configurations according to the specific operational requirements of the work area. It can be configured with up to 3 operating units and 2 transfer compartments, with 2, 4 or 6 sets of hand sleeves to support user work. Larger, more flexible configuration for higher throughput or multiple batch runs. Disinfection performance is guaranteed by Bioquell's recognized hydrogen peroxide vapor technology. "Out of the box" high efficiency cycle for pharmaceutical and biosafety applications.
Each compartment of the equipment (with a cabin pressure of +100Pa to -100pa controllable) - built-in ventilation for rapid disinfection cycles, and the integration of Bioquell's purification technology to produce 35% hydrogen peroxide solution Chemical, rapid disinfection. Create a completely sterile environment inside BioquellQUBE (this sterile environment can be kept for at least 7 days). One module is biologically sterilized while other modules are operational. Help researchers create a relatively clean, sterile microspace (a Class A ISO5 workspace) to meet the environmental requirements of the experimental process.
The flexibility of Bioquell QUBE is not limited to its modular design and hydrogen peroxide technology.
It also offers seamless integration options such as integration with the world's leading Merck & Rab Aseptic Test Pump; four environmental inspection solutions; fast transfer channels for cameras that display and record worksheets and integrated screens that can be set to positive The environment of pressure or negative pressure, and the air circulation of the room, can be operated without accessing the exhaust pipe. Ergonomically designed with an integrated footrest and door opener. Provides excellent "cleanability" using novel plastic molding techniques. It is an ideal alternative to traditional stainless steel isolators.
Please see the detailed video introduction:
Boano China, your loyal partner!
Zhoushan Fudan Tourism CO., LTD , https://www.fudanfood.com
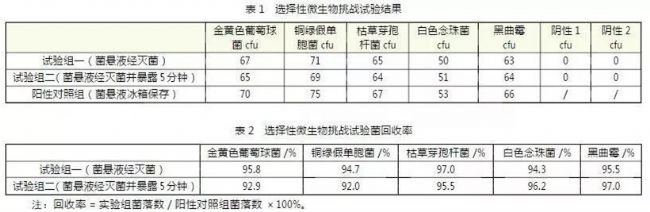 The final sterilization effect of the sterile isolator was verified by hydrogen peroxide gas concentration and distribution status confirmation, BI challenge test, selective microbial challenge test, and microbial detection of the internal environment of the isolator (settlement, floating bacteria, surface microorganisms). Conclusion: After the sterile isolator is sterilized by hydrogen peroxide steam, the microorganisms on the surface of the cabin are killed, the microorganisms inside the article are not affected, and the residual hydrogen peroxide has no effect on the microorganisms. The system of the sterile isolator is extinguished. The effect of the bacteria reached the expected requirements. (
The final sterilization effect of the sterile isolator was verified by hydrogen peroxide gas concentration and distribution status confirmation, BI challenge test, selective microbial challenge test, and microbial detection of the internal environment of the isolator (settlement, floating bacteria, surface microorganisms). Conclusion: After the sterile isolator is sterilized by hydrogen peroxide steam, the microorganisms on the surface of the cabin are killed, the microorganisms inside the article are not affected, and the residual hydrogen peroxide has no effect on the microorganisms. The system of the sterile isolator is extinguished. The effect of the bacteria reached the expected requirements. (