1. The suitable application time of microbial fertilizer is early morning and evening or no rainy and cloudy day, so as to avoid the ultraviolet rays in the sun killing the microorganisms.
2. Microbial fertilizers should be avoided under high temperature and drought conditions. It is necessary to combine measures such as watering the cover soil to prevent the microbial fertilizer from being exposed to direct sunlight or being difficult to function due to insufficient water.
3. Microbial fertilizers cannot be soaked in water for a long time, and they should be irrigated in wet and dry fields to promote biological activities. Products based on aerobic microorganisms should not be used in paddy fields.
4. Microbial fertilizers can be used alone or mixed with other fertilizers, but microbial fertilizers should avoid mixing with unripe farm manure. Mixed with unripe organic fertilizer, it will kill microorganisms at high temperature and affect fertilizer efficiency.
5. Microbial fertilizers should be avoided at the same time as pesticides. Do not use microbial fertilizers mixed with pesticides or fungicides. Microbial fertilizers should not be stored for a long time, and should be used in time after unpacking. It is best to use them all at once.
6. Avoid blind application of microbial fertilizers. Microbial fertilizers mainly provide beneficial microbial flora, rather than providing mineral nutrients. Microbial fertilizers cannot completely replace chemical fertilizers.
Disclaimer: Some articles of this website are transferred from the Internet. If the legal rights of third parties are involved, please inform this website for processing. phone
Intermediates of Cladribine, Carvedilol, Lurasidone, olmesartan, Risedronate Sodium, Atazanavir, Saxagliptin, Dabigatran,Dapoxetine,Cefixime,Ceftaroline fosamil and etc.
In the short span of time, we have emerged as most promising pharmaceutical intermediates manufacturers, chemical intermediates and bulk drug intermediates suppliers. Our consistent supply, quality products and dedication towards clients have opened up many international avenues for our growth.
In addition, the company also can follow the customer's product needs custom synthesis services
MAIN API PRODUCTS USP/BP
|
PRODUCT NAME |
CAS NUMBER |
SPEVIFICATION |
|
Azithromycin |
117772-70-0 |
BEP |
|
Cefpirome Sulphate sterile |
84957-29-9 |
USP JP16 |
|
Ceftriaxone Sodium (Sterile) |
104376-79-6 |
USP31 |
|
Cefotaxime |
64485-93-4 |
USP30 |
|
Ciprofloxacin HCL |
85721-33-1 |
USP/BP |
|
Gentamicin sulphate |
1405-41-0 |
BP |
|
Levofloxacin |
100986-85-4 |
USP27 |
|
Lincomycin Hydrochloride |
859-18-7 |
EP6.0 |
|
Moxifloxacin Hydrochloride |
186826-86-8 |
USP31 |
|
Tigecycline |
220620-09-7 |
USP |
|
Linezolid |
165800-03-3 |
EP |
|
Dexamethasone |
50-02-2 |
USP/BP/EP |
|
Methylprednisolone |
83-43-2 |
USP/BP/EP |
|
Dexketoprofen trometamol |
156604-79-4 |
BP2008 |
|
Ibuprofen |
15687-27-1 |
BP |
|
Metamizol |
68-89-3 |
DAB |
|
Sulindac |
38194-50-2 |
USP/BP/EP |
|
Naproxcinod |
163133-43-5 |
USP28 |
|
Tripelennamine Hydrochloride |
154-69-8 |
USP28 |
|
Itraconazole |
84625-61-6 |
USP/BP |
|
Cytarabine |
147-94-4 |
USP31 |
|
Leucovorin Calcium |
1492-18-8 |
USP32 |
|
Valsartan |
137862-53-4 |
USP30 |
|
Telmisartan |
144701-48-4 |
USP31 |
|
Rosuvastatin Calcium |
147098-20-2 |
USP/BP |
|
Pitavastatin Calcium |
147526-32-7 |
USP/BP |
|
Fluvastatin |
93957-54-1 |
USP31 |
|
Vinpocetine |
42971-09-5 |
EP6.0 |
|
Atazanavir |
198904-31-3 |
BP |
|
Rosiglitazone |
122320-73-4 |
USP30 |
|
Esomeprazole Magnesium |
161973-10-0 |
USP/BP |
|
Topiramate |
97240-79-4 |
USP31 |
|
Fexofenadine HCl |
153439-40-8 |
Inhouse |
|
Bosentan |
147536-97-8 |
Inhouse |
|
D-Cysteine |
921-01-7 |
Inhouse |
|
D-Phenylalanine |
673-06-3 |
Inhouse |
|
Linagliptin |
668270-12-0 |
Inhouse |
|
Rivaroxaban |
366789-02-8 |
USP |
|
Saxagliptin |
361442-04-8 |
USP |
|
Vildagliptin |
274901-16-5 |
USP |
Major Pharmaceutical Intermediates
| Items Descripation | Structure | Application |
|
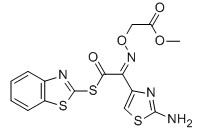
MICA ESTER CAS No: 246035-38-1 Purity: ≥98% |
 |
For Cefixime |
|
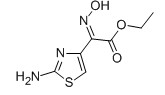
EHATA CAS No: 64485-82-1 Purity: ≥98% |
 |
For Ceftazidine |
|
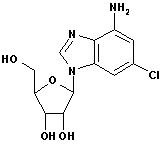
2-Chloroadenine CAS No: 1839-18-5 |
 |
For Cladribine, Fludarabine et al |
|
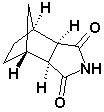
Bicyclo(2,2,1)Heptane-2,3-di-exo-carboximide CAS No: 14805o-29-9 |
 |
For Lurasidne |
|
(R,R)-1,2-Bis(methanesulfonyloxy methyl)Cyclohexane CAS No: 186204-35-3 |
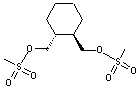 |
For Lurasidone |
|
3-(Piperazin-1-yl)benzol[d] isothiazole CAS No: 87691-87-0 |
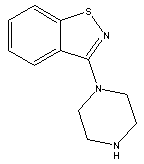 |
For Lurasidone |
|
Trityl olmesartan CAS No: 144690-92-6 Purity: ≥98% |
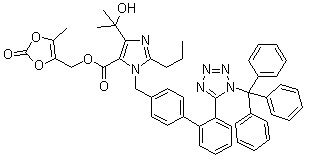
|
For olmesartan |
|
3-Acetyl Pyridine CAS No: 350-03-8 |
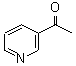
|
For Risedronate Sodium |
|
3-(AceticAcid)pyridine HCL CAS No: 6419-36-9 |
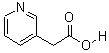 |
For Risedronate Sodium |
|
Risedronic Acid CAS No: 105462-24-6 |
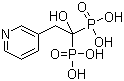 |
For Risedronate Sodium |
|
3-Hydroxy-1-adamantyl-D-Glycine CAS No: 709031-29-8 |
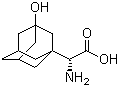 |
For Saxagliptin |
|
(1s,3s,5s)-3-(aminocarbonyl)-2-azabicyclo(3,1,0) hexane-2-carboxylic acid tert-butyl ester CAS No: 361440-67-7 |
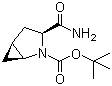 |
For Saxagliptin |
|
(S)-N-Boc-3- hydroxy-adamantylglycine CAS No: 361442-00-4 |
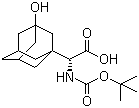 |
For Saxagliptin |
|
2-Azabicyclo[3.1.0] hexane-3-carbonitrile, (1s,3s,5s)- CAS No: 866083-42-3 |
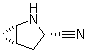 |
For Saxagliptin |
|
Ethyl 3-(pyridin-2-ylamino) propanoate CAS No: 103041-38-9 |
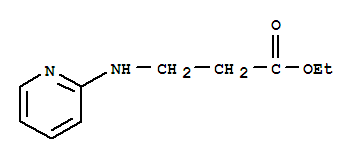 |
For Dabigatran |
|
N-(4-Cyanophenyl) glycine CAS No: 42288-26-6 |
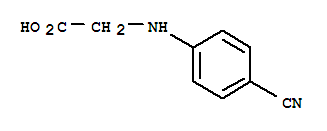 |
For Dabigatran |
|
4-methylamino-3-nitrobenzoic Acid CAS No: 41263-74-5 |
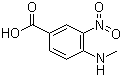 |
For Dabigatran |
|
S-3-Amino-3-phenylpropanoic acid ethyl ester HCL CAS No: 167834-24-4 |
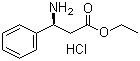 |
For Dapoxetine |
|
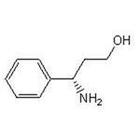
(S)-3-Amino-3-Phemylpropan -1-ol CAS No: 82769-76-4 |
|
For Dapoxetine |
|
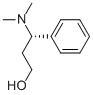
(S)-3-Dimethylamino-3-Phemylpropanol CAS No: 82769-75-3 |
|
For Dapoxetine |
|
4-{4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-butynil}-α,α-dimethyl benzene acetic acid CAS No: 832088-68-3 |
For Fexofenadine HCl | |
|
Methyl 2-(4-(4-chlorobutanoyl)phenyl)-2-methylpropanoate CAS No:154477-54-0 |
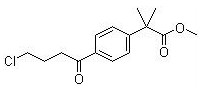
|
For Fexofenadine HCl |
|
5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone CAS No 461432-22-4 |
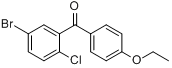
|
For Dapagliflozin |
|
4-(5-Bromo-2-chlorobenzyl)phenyl ethyl ether CAS No :461432-23-5 |
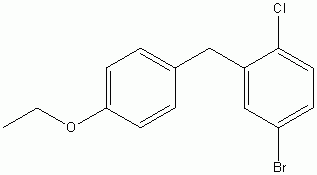
|
For Dapagliflozin |
Mica Ester,Pharma Intermediates,Ciprofloxacin Hcl Uses,Active Pharmaceutical Ingredients
NINGBO VOICE BIOCHEMIC CO. LTD , https://www.medicine-voice.com