In 2012, Emily, a 6-year-old girl with acute lymphoblastic leukemia, tried the research CAR-T cell treatment at Carl June Laboratory without medical treatment. The miracle happened now, and she has been healthy and survived since treatment. CD19 CAR T cell therapy has since entered the public eye and clinical practice at a speed of 100 meters. Among them, CAR, the chimeric antigen receptor, is the core element of this treatment. On July 5th, one of the inventors of CAR-T cells, Professor Car June, who is known as the father of CAR T cells, published a paper in the internationally renowned journal NEJM to discuss a brief history of the development of CAR-T cell therapy. The effectiveness and toxic effects of existing CAR-T treatments, and prospects for the future development and challenges of CAR-T treatment. This issue will be presented to everyone!


Dr. Carl June (image source parkerici.org)
CAR-T cell therapy is a typical tumor immunotherapy. Cancer immunotherapy aims to treat cancer by increasing the body's immune response to tumor cells. As a new therapeutic strategy, it has been successful in the treatment of metastatic cancer as a combination of chemotherapy, surgery, radiotherapy and small molecule targeted drugs. Immuno-oncology drugs cover a wide range of drugs, including vaccines, cytokines, oncolytic viruses, bispecific molecules, and cell therapy. Genetically engineered T cells are a new class of therapeutic drugs, and treatments based on chimeric antigen receptor (CAR) T cells are currently approved by the FDA for the treatment of leukemias and lymphomas.
Video (How does CAR T therapy work)
The core challenge of immuno-oncology is that most tumor antigens are autoantigens and can be expressed in normal tissues. Due to the body's self-protection mechanism, this type of antigen is often unable to effectively activate the immune system, so the anti-tumor immune response is short-lived. The efficacy is poor, because the evolution of the host immune response tends to prevent autoimmunity, and genetic engineering that directly edits the genes of T cells can help overcome this immune tolerance.
Structure of CARs
CARs are artificially synthesized, and artificial receptors are not found in nature. The specificity, function and metabolism of T cells can be directly reshaped by implanting T cells by gene editing technology. CARs include a T cell activation region and a single-chain antibody (scFv) extracellularly derived from immunoglobulin heavy and light chains, which are specifically associated with T cells. The above structure is called a generation of CARs, and non-HLA-dependent recognition of antigens cannot achieve sustained T cell responses due to limited signal capabilities.
Chimeric costimulatory receptors linked to single-chain antibodies increase T cell proliferation and anti-apoptotic ability and are the basis for the success of dual-signal CARs. Functional T-cells can be efficiently amplified after repeated antigen exposure. The second generation of CARs, which produce a sustained effect, is the basis of modern CAR-T cell therapy.
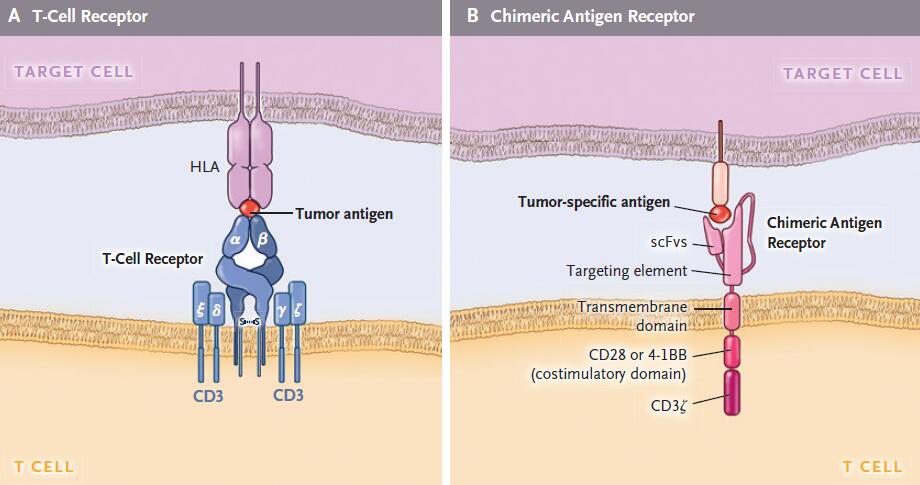
T cell receptor and CAR structure
CD19 CAR-T is currently the most successful cell therapy <br> CAR-T targeting CD19 is currently the most successful and most widely used cell therapy. CD19 was chosen as a target because it is frequently expressed in B-cell leukemia and lymphoma, and its expression level and level are much higher than other potential targets such as CD20 and CD22. CD19 can also be expressed in normal B cells, so it can cause B cell hyperplasia. The effect of B cell reduction on humoral immunity can be alleviated by infusion of immunoglobulin. B cell hyperplasia precedes the body's antibody response to CAR.
The 2017 FDA approved a study of CD19 CAR-T treatment in which gamma retrovirus or lentiviral vectors were used to encode CARs containing CD28 or 4-1BB costimulatory molecules. Clinical studies have quickly shown the effectiveness of the drug in relapsed refractory B-cell tumors, and the treatment response is sustainable.
At present, there is a lack of head-to-head research on various CAR-T cell therapies, but based on the existing research results, the following conclusions can be drawn: drugs containing mouse sequences can trigger host immunity leading to drug failure, and lack of immunogenicity can make CAR-T The cells persist and improve the recurrence of leukemia, so the CAR design is preferred with a fully humanized sequence. In addition, there have been reports that CARs using CD28 costimulatory factors have a potential neurotoxic risk, but further research is needed to confirm the association between the two.
The toxic effects of CAR-T cell therapy are still not negligible <br> All cancer treatments have side effects, and CAR-T cells are no exception. The toxicity is mainly from off-target, and the specificity and T of the antibody single-chain variable region fragment. Related to cell activation. When the target cells are cleared or the implanted CAR-T cells disappear, the side effects disappear, which is completely different from the off-target toxicity of traditional cytotoxic chemotherapy. Traditional chemotherapy toxicity can lead to permanent genetic changes in tissue cells, even involving stem cells, affecting long-term clinical outcomes.
B cell hyperplasia is a predictable adverse reaction because CARs target B cell differentiation antigens. Clinical studies have shown that CD19 CARs-induced B cell hyperplasia is more thorough than rituximab, which can be quickly recovered after CAR-T cell removal. Treatment guidelines for CAR-induced B cell hyperplasia continue to improve, and adult and child treatments are not the same because children have incomplete long-lived plasma cells and weaker humoral immunity.
In addition, in the treatment of CAR-T cells, some patients have fever, hypoxemia, hypotension, and nervous system changes, accompanied by an increase in cytokines. These changes are called cytokine release syndrome (CRS), which occurs with CD19 and B. Cell Mature Antigen (BCMA) CARs are associated. In the case of CD19 CARs, the severity of CRS is associated with tumor burden and may even develop into a life-threatening capillary leak syndrome. There is little CRS in patients who do not respond to CAR treatment. CRS occurs in association with T cell activation and high levels of cytokines such as interferon gamma and IL-6. Totizumab is an IL-6 receptor antagonist that has a therapeutic effect on severe CRS and has been approved for use by the FDA. If the blockade of IL-6 receptors still does not relieve symptoms quickly, it should be treated with glucocorticoids immediately.
All CAR-T cell treatments against CD19 and BCMA are neurotoxic, and bispecific anti-CD19 and CD3 monoclonal antibody blinatumomab also have similar neurotoxicity, so it is speculated that the occurrence of neurotoxicity is related to the CD19 target antigen. Although the specific mechanism of neurotoxicity is not clear, the toxicity is usually completely reversible and has no relationship with the central nervous system (CNS) metastasis. Cerebral edema can occur when neurotoxicity occurs. The current treatment is still an empirical treatment. Because of CRS risk and neurotoxicity, FDA requires that physicians and hospital staff complete training on side-effect therapy before treatment with CAR-T cells.
The integration of viral vectors into the human body is also a safety issue that needs attention. More than 1000 patients have been treated with T cell receptors or CAR-adjusted T cells, and tumor transformation has not yet occurred.
Other types of CAR treatment
CD19 CAR-T treatment is currently the most successful and well-known CAR-T treatment, from which some results can be drawn: B cell hyperplasia indicates that CAR-T cells can also cause damage to normal tissues, although CD19 CAR-T treatment This side effect is not life-threatening, but other side effects of other targets may be life-threatening; CD19 CAR-T treatment is completely negative after relapse, CD19 is negative, indicating the presence of antigen escape; targeting CD19 is superior to CD20 and CD22, suggesting that high-density CAR target expression is more effective; some extramedullary diseases, such as posterior peritoneal or CNS leukemia, do not respond to treatment, lack of formal assessment data on the response rate of extramedullary disease; it is unclear why CD19 of acute lymphoblastic leukemia The CAR-T response is significantly better than chronic lymphocytic leukemia or non-Hodgkin's lymphoma, and the disease site, tumor microenvironment, and host T cell function may be associated with it.
The efficacy of CAR treatment for B-cell tumors provides an example of the treatment of other malignant hematologic tumors. It has now begun to explore CAR targets for multiple myeloma, such as kappa light chain, CD138, Lewis Y antigen, BCMA, CS1, CD38. And integrin beta, in which the results of targeting BCMA are very good. CAR targets for acute myeloid leukemia are also being explored, including CD33, CLEC12A, CD44v6, EMR2, Tim3, CD70, Lewis Y antigen, CD123, and folate receptor beta, in which CD123 CAR-T cells can cause fatal complications. Therefore, this target needs to be carefully evaluated. For diseases lacking a high-quality target like CD19, targeting ≥2 antigens at the same time has a certain effect on reducing antigen escape without aggravating toxicity.
Cell Engineering and Synthetic Biology <br> The combination and development of genetic engineering and synthetic biology has made it possible to create more functional T cells: combining target T cells, recognizing two antigens, increasing the effectiveness of treatment and Safety; engineered T cells can enter the tumor microenvironment as a carrier; further improve the safety of T cells through a controlled suicide switch; nuclease provides a new method for T cell gene knockout or transgenesis. In short, the development and integration of technology provides unlimited possibilities for the development of more functional cells.
Challenge and future
CAR-T cell therapy still needs to be explored in solid tumors
CD19 CAR-T cells are the first commercially approved transgenic therapy. Genetically engineered T cells as the precise treatment of cancer, the most important scientific challenge is how CAR-T cells treat solid tumors. The most prominent example is the intracranial use of IL13RαCAR T cells for the treatment of multiple brain malignant gliomas, resulting in significant tumor retraction. However, in the treatment of other solid tumors, it is still in the exploratory stage, not only to have a significant effect, but also to prevent or reduce related side effects.
Versatile CAR-T is the future direction <br> The enormous cost and therapeutic complexity of autologous CAR-T cell therapy limits its widespread use. Currently, a wide range of gene-editing technologies have been developed to develop universally applicable CAR-T cells and have been successfully treated in several children with acute pro-B-lymphocytic leukemia. However, the most common challenge for T cells is how to avoid host anti-grafts. Reaction and graft versus host response. Using human embryonic stem cells and inducible pluripotent stem cells as T cell sources, it is possible to produce T cells with many excellent traits, including antigen specificity, no allogeneic reaction, histocompatibility and functional enhancement. .
Forming Business Models <br> The approval of CD19 CAR-T cells for the treatment of tumors has facilitated the transformation of engineered cells to treat other diseases. There are more than 250 clinical trials of CAR-T cell therapy registered on ClinicalTrials.gov worldwide, most of which are concentrated in the United States and China, and a small number in Europe and Japan. In conclusion, it can be foreseen that CAR-T cells, as a breakthrough therapy for cancer treatment, are supported by national policies, under the huge talent dividend, plus the high plasticity of the cell carrier itself, especially the combination of gene editing technology and cell therapy. The development of the product UCAR-T (Universal CAR-T) is expected to achieve "off the shelf" of the same batch of mass-sized cell drugs faster and more efficiently, which will drive the cell therapy industry faster. The road to change.
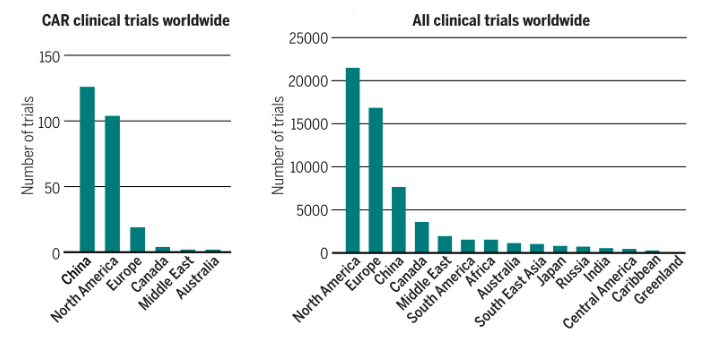
CAR-T clinical research is distributed globally (Source: Science)
references
June, Carl H., and Michel Sadelain. "Chimeric Antigen Receptor Therapy." New England Journal of Medicine 379.1 (2018): 64-73.
June CH, Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer [J]. Science, 2018, 359 (6382): 1361-1365.


Dr. Carl June (image source parkerici.org)
CAR-T cell therapy is a typical tumor immunotherapy. Cancer immunotherapy aims to treat cancer by increasing the body's immune response to tumor cells. As a new therapeutic strategy, it has been successful in the treatment of metastatic cancer as a combination of chemotherapy, surgery, radiotherapy and small molecule targeted drugs. Immuno-oncology drugs cover a wide range of drugs, including vaccines, cytokines, oncolytic viruses, bispecific molecules, and cell therapy. Genetically engineered T cells are a new class of therapeutic drugs, and treatments based on chimeric antigen receptor (CAR) T cells are currently approved by the FDA for the treatment of leukemias and lymphomas.
Video (How does CAR T therapy work)
The core challenge of immuno-oncology is that most tumor antigens are autoantigens and can be expressed in normal tissues. Due to the body's self-protection mechanism, this type of antigen is often unable to effectively activate the immune system, so the anti-tumor immune response is short-lived. The efficacy is poor, because the evolution of the host immune response tends to prevent autoimmunity, and genetic engineering that directly edits the genes of T cells can help overcome this immune tolerance.
Structure of CARs
CARs are artificially synthesized, and artificial receptors are not found in nature. The specificity, function and metabolism of T cells can be directly reshaped by implanting T cells by gene editing technology. CARs include a T cell activation region and a single-chain antibody (scFv) extracellularly derived from immunoglobulin heavy and light chains, which are specifically associated with T cells. The above structure is called a generation of CARs, and non-HLA-dependent recognition of antigens cannot achieve sustained T cell responses due to limited signal capabilities.
Chimeric costimulatory receptors linked to single-chain antibodies increase T cell proliferation and anti-apoptotic ability and are the basis for the success of dual-signal CARs. Functional T-cells can be efficiently amplified after repeated antigen exposure. The second generation of CARs, which produce a sustained effect, is the basis of modern CAR-T cell therapy.

T cell receptor and CAR structure
CD19 CAR-T is currently the most successful cell therapy <br> CAR-T targeting CD19 is currently the most successful and most widely used cell therapy. CD19 was chosen as a target because it is frequently expressed in B-cell leukemia and lymphoma, and its expression level and level are much higher than other potential targets such as CD20 and CD22. CD19 can also be expressed in normal B cells, so it can cause B cell hyperplasia. The effect of B cell reduction on humoral immunity can be alleviated by infusion of immunoglobulin. B cell hyperplasia precedes the body's antibody response to CAR.
The 2017 FDA approved a study of CD19 CAR-T treatment in which gamma retrovirus or lentiviral vectors were used to encode CARs containing CD28 or 4-1BB costimulatory molecules. Clinical studies have quickly shown the effectiveness of the drug in relapsed refractory B-cell tumors, and the treatment response is sustainable.
At present, there is a lack of head-to-head research on various CAR-T cell therapies, but based on the existing research results, the following conclusions can be drawn: drugs containing mouse sequences can trigger host immunity leading to drug failure, and lack of immunogenicity can make CAR-T The cells persist and improve the recurrence of leukemia, so the CAR design is preferred with a fully humanized sequence. In addition, there have been reports that CARs using CD28 costimulatory factors have a potential neurotoxic risk, but further research is needed to confirm the association between the two.
The toxic effects of CAR-T cell therapy are still not negligible <br> All cancer treatments have side effects, and CAR-T cells are no exception. The toxicity is mainly from off-target, and the specificity and T of the antibody single-chain variable region fragment. Related to cell activation. When the target cells are cleared or the implanted CAR-T cells disappear, the side effects disappear, which is completely different from the off-target toxicity of traditional cytotoxic chemotherapy. Traditional chemotherapy toxicity can lead to permanent genetic changes in tissue cells, even involving stem cells, affecting long-term clinical outcomes.
B cell hyperplasia is a predictable adverse reaction because CARs target B cell differentiation antigens. Clinical studies have shown that CD19 CARs-induced B cell hyperplasia is more thorough than rituximab, which can be quickly recovered after CAR-T cell removal. Treatment guidelines for CAR-induced B cell hyperplasia continue to improve, and adult and child treatments are not the same because children have incomplete long-lived plasma cells and weaker humoral immunity.
In addition, in the treatment of CAR-T cells, some patients have fever, hypoxemia, hypotension, and nervous system changes, accompanied by an increase in cytokines. These changes are called cytokine release syndrome (CRS), which occurs with CD19 and B. Cell Mature Antigen (BCMA) CARs are associated. In the case of CD19 CARs, the severity of CRS is associated with tumor burden and may even develop into a life-threatening capillary leak syndrome. There is little CRS in patients who do not respond to CAR treatment. CRS occurs in association with T cell activation and high levels of cytokines such as interferon gamma and IL-6. Totizumab is an IL-6 receptor antagonist that has a therapeutic effect on severe CRS and has been approved for use by the FDA. If the blockade of IL-6 receptors still does not relieve symptoms quickly, it should be treated with glucocorticoids immediately.
All CAR-T cell treatments against CD19 and BCMA are neurotoxic, and bispecific anti-CD19 and CD3 monoclonal antibody blinatumomab also have similar neurotoxicity, so it is speculated that the occurrence of neurotoxicity is related to the CD19 target antigen. Although the specific mechanism of neurotoxicity is not clear, the toxicity is usually completely reversible and has no relationship with the central nervous system (CNS) metastasis. Cerebral edema can occur when neurotoxicity occurs. The current treatment is still an empirical treatment. Because of CRS risk and neurotoxicity, FDA requires that physicians and hospital staff complete training on side-effect therapy before treatment with CAR-T cells.
The integration of viral vectors into the human body is also a safety issue that needs attention. More than 1000 patients have been treated with T cell receptors or CAR-adjusted T cells, and tumor transformation has not yet occurred.
Other types of CAR treatment
CD19 CAR-T treatment is currently the most successful and well-known CAR-T treatment, from which some results can be drawn: B cell hyperplasia indicates that CAR-T cells can also cause damage to normal tissues, although CD19 CAR-T treatment This side effect is not life-threatening, but other side effects of other targets may be life-threatening; CD19 CAR-T treatment is completely negative after relapse, CD19 is negative, indicating the presence of antigen escape; targeting CD19 is superior to CD20 and CD22, suggesting that high-density CAR target expression is more effective; some extramedullary diseases, such as posterior peritoneal or CNS leukemia, do not respond to treatment, lack of formal assessment data on the response rate of extramedullary disease; it is unclear why CD19 of acute lymphoblastic leukemia The CAR-T response is significantly better than chronic lymphocytic leukemia or non-Hodgkin's lymphoma, and the disease site, tumor microenvironment, and host T cell function may be associated with it.
The efficacy of CAR treatment for B-cell tumors provides an example of the treatment of other malignant hematologic tumors. It has now begun to explore CAR targets for multiple myeloma, such as kappa light chain, CD138, Lewis Y antigen, BCMA, CS1, CD38. And integrin beta, in which the results of targeting BCMA are very good. CAR targets for acute myeloid leukemia are also being explored, including CD33, CLEC12A, CD44v6, EMR2, Tim3, CD70, Lewis Y antigen, CD123, and folate receptor beta, in which CD123 CAR-T cells can cause fatal complications. Therefore, this target needs to be carefully evaluated. For diseases lacking a high-quality target like CD19, targeting ≥2 antigens at the same time has a certain effect on reducing antigen escape without aggravating toxicity.
Cell Engineering and Synthetic Biology <br> The combination and development of genetic engineering and synthetic biology has made it possible to create more functional T cells: combining target T cells, recognizing two antigens, increasing the effectiveness of treatment and Safety; engineered T cells can enter the tumor microenvironment as a carrier; further improve the safety of T cells through a controlled suicide switch; nuclease provides a new method for T cell gene knockout or transgenesis. In short, the development and integration of technology provides unlimited possibilities for the development of more functional cells.
Challenge and future
CAR-T cell therapy still needs to be explored in solid tumors
CD19 CAR-T cells are the first commercially approved transgenic therapy. Genetically engineered T cells as the precise treatment of cancer, the most important scientific challenge is how CAR-T cells treat solid tumors. The most prominent example is the intracranial use of IL13RαCAR T cells for the treatment of multiple brain malignant gliomas, resulting in significant tumor retraction. However, in the treatment of other solid tumors, it is still in the exploratory stage, not only to have a significant effect, but also to prevent or reduce related side effects.
Versatile CAR-T is the future direction <br> The enormous cost and therapeutic complexity of autologous CAR-T cell therapy limits its widespread use. Currently, a wide range of gene-editing technologies have been developed to develop universally applicable CAR-T cells and have been successfully treated in several children with acute pro-B-lymphocytic leukemia. However, the most common challenge for T cells is how to avoid host anti-grafts. Reaction and graft versus host response. Using human embryonic stem cells and inducible pluripotent stem cells as T cell sources, it is possible to produce T cells with many excellent traits, including antigen specificity, no allogeneic reaction, histocompatibility and functional enhancement. .
Forming Business Models <br> The approval of CD19 CAR-T cells for the treatment of tumors has facilitated the transformation of engineered cells to treat other diseases. There are more than 250 clinical trials of CAR-T cell therapy registered on ClinicalTrials.gov worldwide, most of which are concentrated in the United States and China, and a small number in Europe and Japan. In conclusion, it can be foreseen that CAR-T cells, as a breakthrough therapy for cancer treatment, are supported by national policies, under the huge talent dividend, plus the high plasticity of the cell carrier itself, especially the combination of gene editing technology and cell therapy. The development of the product UCAR-T (Universal CAR-T) is expected to achieve "off the shelf" of the same batch of mass-sized cell drugs faster and more efficiently, which will drive the cell therapy industry faster. The road to change.

CAR-T clinical research is distributed globally (Source: Science)
references
June, Carl H., and Michel Sadelain. "Chimeric Antigen Receptor Therapy." New England Journal of Medicine 379.1 (2018): 64-73.
June CH, Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer [J]. Science, 2018, 359 (6382): 1361-1365.
Brine Peeled Garlic,Fresh Garlic,Brine Peeled Garlic Packet,Brine Peeled Garlic 5 Lbs
shandong changrong international trade co.,ltd. , https://www.cragriculture.com