When western blots are used for quantification, many believe that only the protein of interest needs to be imaged, stripped, re-incubated with the internal reference protein, and then used for normalization, regardless of linear range detection. However, there are several misunderstandings related to this, and more and more journals now require an appropriately defined standardization process, including proof of linear testing, and all drafts.
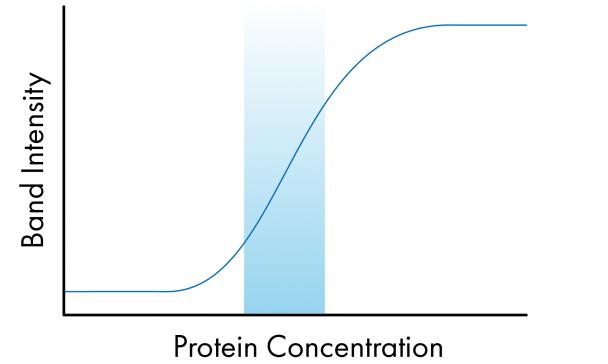
Linear detection is the area in which the band intensity is proportional to the target protein on the membrane. As the amount of protein increases, the band strength should increase. In the image below, you can see where the problem is, the linear range is marked with a blue box, and outside of this area, the proportional relationship is lost, especially when the signal is completely supersaturated or very low.
 Quantification of Western Blots Western blot quantification is truly accurate when both the target protein and the loading control have similar linear ranges, so things become more complicated when the loading control is considered. This is shown in the two figures below; where the first detected linear range overlaps, the quantification of protein within this overlap region will be good. However, in the latter, they do not overlap and it is easy to see how changes in protein concentration affect the intensity of one protein band without affecting the band strength of the other protein. In this case, the quantification is not accurate.
Quantification of Western Blots Western blot quantification is truly accurate when both the target protein and the loading control have similar linear ranges, so things become more complicated when the loading control is considered. This is shown in the two figures below; where the first detected linear range overlaps, the quantification of protein within this overlap region will be good. However, in the latter, they do not overlap and it is easy to see how changes in protein concentration affect the intensity of one protein band without affecting the band strength of the other protein. In this case, the quantification is not accurate. 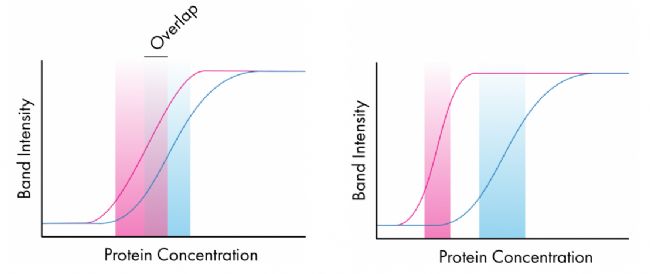
Determining the linear range is relatively easy and can be achieved by sample and serial dilution. If the linear ranges overlap, it is equally easy to determine the amount of sample to load. However, if the ranges do not overlap, as is the very common low-abundance protein associated with the evaluation of highly expressed housekeeping genes, quantification becomes difficult. Also, if the protein you are interested in is stimulated to rise or fall, then this may further interfere with your attempts to adapt to the linear range.
What should we do if your linear ranges do not overlap?
However, there are several ways to do it. The often overlooked step is to change the quality control. If you know that your protein expression level is relatively low, then loading control options such as beta-actin may not be appropriate. In these cases, it may be more beneficial to use organelle-specific proteins, such as Lamin B1 or HSP60, because they should not be expressed particularly high, but care should be taken to confirm that these proteins are expressed and will not change due to processing.
The second option is to shift from single internal reference quality control to total protein quantification as a means of quality control. A wider range of dynamics is produced by assessing the entire protein range. These stains include gel and membrane staining and can be imaged in a variety of different ways. Furthermore, as mentioned earlier, this technique is a very good method for checking the quality of protein separation and transfer efficiency.
Finally, the dynamic range can be extended during imaging. The problem of poor film dynamic range and inaccurate exposure time can make things more complicated. Gels and membranes are imaged inside the imaging system, such as our c-series. Imaging systems such as the c600 and the Azure Sapphire dual-mode multispectral laser imaging system enable imaging over a wider dynamic range, enabling linear range detection.
Insulin Syringes Needle,Disable Syringe,Monoject Syringe,10 Ml Syringe
FOSHAN PHARMA CO., LTD. , https://www.fs-pharma.com