Release date: 2016-09-20
According to the World Health Organization, more than 640 million people worldwide will suffer from diabetes by 2040. Most of the newly added patients are concentrated in the western Pacific region such as China and Southeast Asia. A large population means a big market. Last week in Munich, Germany, this year's annual meeting of the European Diabetes Research Association, a clear trend at this year's annual meeting: from Novartis to Sanofi to Novo Nordisk, the traditional pharmaceutical or device manufacturers attending the meeting began to bet Pressed on new technology, it can be said that black technology is frequent. Everyone hopes to survive in the competition.
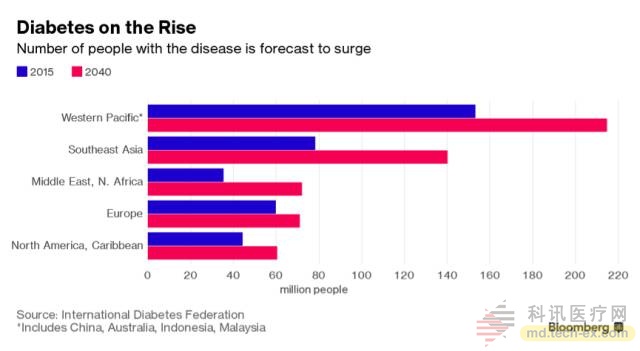
Today's diabetes drug market is highly competitive, and both insulin injection devices, GLP-1 receptor agonists, and oral new hypoglycemic agents are looking for new growth breakthroughs.
The industry's leading boss, Novo Nordisk, is investing in oral diabetes drugs that do not require injections, while Sanofi and Google's parent company, Alphabet, are preparing to invest about $500 million to explore new combinations of software and drugs to help patients control disease. There are other researchers working on artificial pancreas to replace dysfunctional organs and delivering insulin through "smart patches" to help patients control blood sugar levels.
“The industry is now becoming more and more crowded, and in order to stand out from it, it’s clear that the best insulin production alone will not allow the company to go further,†said Stefan Oelrich, head of Sanofi’s global diabetes business, in an interview. “You have to do more. I think integrating patient data over time to come up with more personalized treatments will be a future trend.â€
Not just about new drugs, but also how to deliver drugs. Insulin pens and related apps developed by Companion Medical of San Diego, Calif., have just passed FDA approval in July. The first FDA-approved smart insulin pen can be used in Eli Lilly's best-selling drug, Humalog. And Novo's best-selling insulin drug, NovoLog, allows patients to track and calculate insulin measurements while sending information to health care workers.

InPen becomes the first intelligent insulin injection device approved by the FDA
Researchers from the University of North Carolina at Chapel Hill and North Carolina State University also said in March that they have developed a patch that covers many tiny needles that can secrete insulin to control blood sugar levels according to patient needs, but further trials are needed. Verification. At the same time, Medtronic is also applying for permission for the first human pancreas in the United States. This device is only mobile phone-sized and can be wirelessly connected to insulin pumps and glucose monitors. The instrument can replace insulin secretion in the human pancreas.
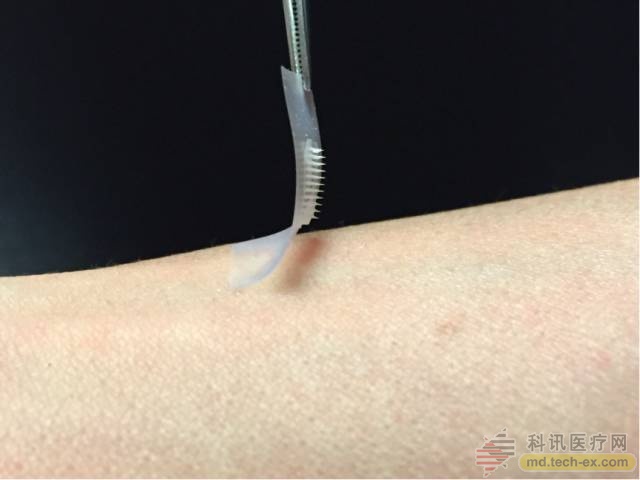
The “smart insulin patch†developed by the University of North Carolina can sense and regulate insulin levels in the body. The developer is a Chinese scientist Gu Zhen.
“There is a great need to understand diabetes and treat diabetes,†said Simon O`Neill, director of health intelligence at Diabetes UK. “We already have a lot of cheap generic drugs, but we can think of better solutions. If pharmaceutical manufacturers can develop products that make people's lives easier, it will be possible to get a huge market."
In fact, the diabetes industry has long sought ways to replace insulin injections. Novo is currently conducting an in-depth test of an oral version of the GLP-1 receptor agonist semaglutide, which stimulates the pancreas but has only been administered by injection until now. On February 23 this year, Norderold announced the success of the fifth phase IIIa trial of the SUSTAIN study. Novo Nordisk also plans to launch a phase III trial of oral semaglutide in 2016. Some even predict that oral semaglutide will become a heavyweight with annual sales of more than $10 billion. If semaglutide can be extended to indications such as weight loss and NASH, It will be a heavyweight of $20 billion.
Roy Eldor, chief medical officer of Israel's Oramade Pharmaceuticals, said the company is now moving in a similar direction, hoping that their own oral insulin will be able to enter the final phase of testing next year.
“The only way to get yourself out of price pressure is to introduce new and better products over the long term,†said Lars Rebien Soerensens, the outgoing CEO of Novo Nordisk, in an interview last month. "Our strategy is innovation."
Novo admitted that many attempts to develop insulin tablets eventually failed. A successful tablet must not only withstand the acidic environment of the digestive tract, but also enter the bloodstream through the filtration of the intestinal wall. Soerensen called oral treatments "the largest research area in the company's history." His successor, Lars Fruergaard Joergensen, said investors should not expect major changes in strategy.
If Novo can overcome these challenges, they will gain a sharp edge and resolve some of the pressures they face, said Kim Nielsen, a fund manager at Carnegie Asset Management in Copenhagen, who is also an investor in Novo. Novo has said it has experienced unprecedented competition in the largest market in the United States, and the company revealed last month that they lost a big contract for NovoLog.
"Oral insulin is a major breakthrough," Nielsen said. “If anyone can successfully bring a good quality and guaranteed product to the market, it will be a big leap in the convenience of diabetes treatment.â€
In the forefront of convenience, France's Sanofi Inc. invested $248 million in Google's Verily Life Sciences LLC to develop a new technology that allows patients and their doctors to respond more quickly to blood sugar fluctuations and avoid diabetes Long-term complications such as heart disease and cancer caused by poor management. Prior to this, Google and Novartis had reached an agreement to develop invisible eyes that use microsensors to read glucose levels in tears.
According to the World Health Organization, the number of people with diabetes has almost quadrupled since the 1980s with increased risk factors such as overweight and obesity. Complications of diabetes can lead to heart disease, stroke, blindness, kidney failure and lower extremity amputation.
At a conference in Munich, Novo will disclose all data on a cardiovascular test for semaglutide drugs, allowing analysts and doctors to determine whether the drug is better than Lilly in preventing heart disease and stroke. . A new Sanofi product can be injected once a day, which contains two drugs that help offset the impact of the Lantus insulin imitation on Sanofi.
"We have been discussing this technology for a long time, and we are now waiting for the drug mechanism to be revealed," said Oramed's Eldor. “I think we will see tremendous progress, not only in our understanding of the origin of diabetes, but also in our ability to treat this disease.â€
In summary, multinational pharmaceutical companies have made efforts to lay out long-acting GLP-1 receptor agonists, oral GLP-1 receptor agonists and oral insulin market. The future competition in the diabetes market will only intensify. It can be said that who is the first to develop Revolutionary drugs or products, who can first attack each other's life!
Source: Health Point
Flex Duct Tape,White Flex Tape,White Flex Seal Tape,Flex Seal Waterproof Tape
Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.jerrytapes.com