Choose the right column
particle. Protein and polypeptide separation is achieved by interaction with the hydrophobic surface of the column-filled particles.
The packed particles in the column are usually based on silica gel because the stability of the silica gel is high and can be stabilized under most solvent conditions (except for pH greater than 6.5 ). In addition, silica gel can be formed into various sizes with different diameters. Porous spherical particles.
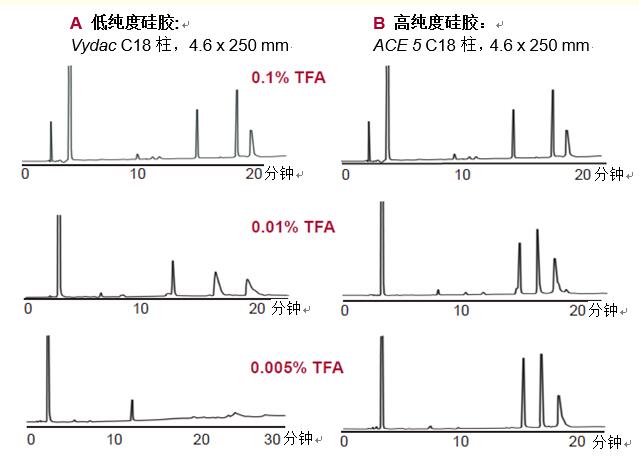
Silica gel purity. The purity of the silica gel filler used in high performance liquid chromatography columns is critical to the separation performance. Metal ion impurities can lead to degradation of resolution and peak tailing, 7A shown (0.01% and 0.005% TFA) in FIG.
Silica gels containing metal ion impurities (Fig. 7A ) require a high concentration of ion pairing reagents such as trifluoroacetic acid ( TFA ) to maintain good peak shape.
Figure 7. Silica gel purity affects the peak shape of the peptide, especially when the concentration of the added ion pair reagent is low. The concentration of ion-pairing reagent required for high-purity silica gel is much lower than that of low-purity silica gel.
Eluent: TFA was added as shown, eluting with a gradient of 10%-55% acetonitrile (ACN) for 37.5 minutes.
Low concentrations of TFA can result in poor peak shape and reduced resolution. When the purity of the silica gel is high (Fig. 7B), a low concentration of TFA of 0.005% produces a good peak shape. This is especially important in liquid chromatography-mass spectrometry because TFA causes a weakening of the signal when using an electrospray interface. A low concentration of TFA in liquid chromatography-mass spectrometry results in a better detection signal.
Aperture. In general, the effect of separating proteins by small pores (~100 angstroms) silica gel in reversed-phase high performance liquid chromatography is poor. Macroporous silica gel (~300 angstrom diameter) allows for better protein separation (Reference 4).
As shown in Figure 8, the protein does not enter the pores and only interacts with the very small outer surface to achieve separation.
Macroporous silica allows proteins, even larger peptides, to enter the pores, allowing full interaction with the hydrophobic interface, resulting in better final peak shape and higher resolution. Today, macroporous silica has been commonly used in the separation of proteins.
The small molecule peptide produced by protease hydrolysis or the like can enter the pores of the small pore silica gel to interact with the hydrophobic interface, so the small pore silica gel can also be used for the separation of protein hydrolyzate.
However, macroporous silica gel also separates peptides well and has different selectivity and resolution.
Figure 8. Reversed-phase high performance liquid chromatography (left) commonly used small pores (~100 angstroms) particles allow only a small amount of protein to enter the pores, thus limiting surface interactions. Macroporous particles (~300 angstroms, right) allow proteins to enter the pores to interact with the hydrophobic interface. 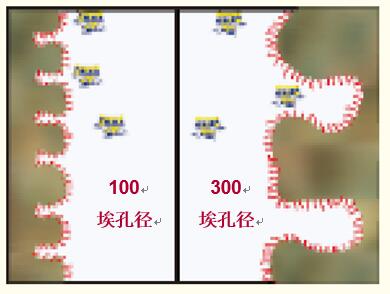
Hydrophobic interface. The silica gel is modified with hydrocarbon molecules to form a hydrophobic interface.
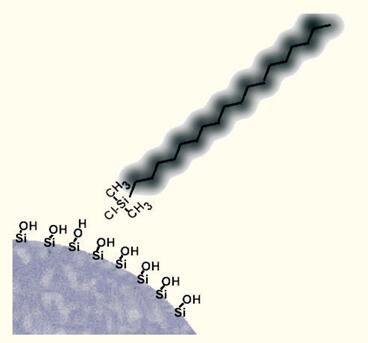
Hydroxysilanes containing hydrocarbon chains, such as octadecylchlorosilane, react with silica gel (with polar silanol groups on the surface) to attach hydrocarbons to the surface of the silica gel (Figure 9).
Due to the steric hindrance effect, the organosilane molecules react only with the silanol groups on the surface of the silica gel, so a large amount of polar silanol groups remain on the surface of the silica gel.
During the "capping" process, the smaller organosilanes are sequentially reacted with polar silanol groups to reduce the amount of polar silanol groups on the silica surface.
Select the separation surface. The chemical process used to modify the surface of silica gel allows a variety of organic groups to adhere to the surface of the silica gel.
The most common modification is the bonding of an eighteen carbon linear fatty chain to form a "C18" column or an ODS column (Fig. 10A).
As shown, the organochlorosilane reacts with most of the silanol groups, but still partially does not react, which forms a thicker layer of hydrocarbon on the surface of the silica gel.
Proteins and polypeptides can be adsorbed to the hydrocarbon layer.
The C18 column is particularly useful for the separation of polypeptides having a molecular weight of less than 2 to 3000 Daltons, and is generally a separation of polypeptides produced by proteolytically hydrolyzed proteins (see pages 26-31) and selection columns for the isolation of natural and synthetic peptides.
The hydrophobic phase formed by butyl bonding to the surface of the silica gel was less (Fig. 10B).
The butyl phase is most suitable for protein separation, but can also be used to separate macromolecular peptides or hydrophobic peptides.
Figure 9. A hydrophobic interface is formed by chemically bonding a hydrophobic ligand to the surface of a silica gel using an organochlorosilane.
Proteins can be separated by a C18 column, but some proteins use a C18 column to separate peak shapes or tailing peaks, so protein separation is recommended for C4 columns.
Other columns for peptide separation include phenyl columns (Ref. 6), which are similar in hydrophobicity to C4 columns and are polar-embedded or polar-terminated columns that enhance the interaction of peptides with silica gel particles. .
Therefore, the polypeptide has different selectivity.
Peptide selectivity. The selectivity of the column for the polypeptide is influenced by the nature and characteristics of the bonding phase and the surface of the underlying silica gel. Different reverse phase columns have different polypeptide selectivities. especially:
The number of phases on the surface of the silica gel (carbon loading) affects selectivity. When the amount of hydrocarbons bonded to the surface of the silica gel is low (lower carbon loading), the polar silanol groups have a greater influence on the separation than the column with a higher carbon loading, thus causing a difference in selectivity.
Different manufacturing processes produce silica gels of different properties, which affect the selectivity of the polypeptide.
Figure 10.
| A C18 hydrophobic column is excellent for separation of peptides with molecular weights less than 2000-3000 Daltons. | 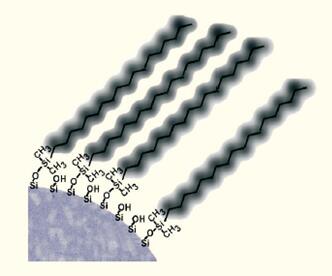 |
| The B C4 hydrophobic column is excellent for the separation of peptides and proteins with molecular weights greater than 3000 Daltons. | 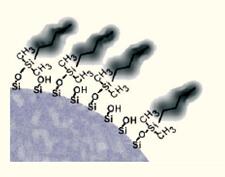 |
Column length. The more interaction between the small molecule and the surface of the silica particle, the higher the resolution; the longer the resolution of the long column than the short column.
However, the protein adsorbed near the top of the column is then eluted, and the interaction with the surface of the silica gel is no longer apparent after elution (Figure 11).
Although the data show that there is still some interaction between the protein and the surface of the silica gel particles, this interaction is not selective and does not improve the resolution between proteins.
The length of the column has no effect on the separation of the protein, and the separation effect of the short column and the long column is the same.
Since the interaction of the polypeptide with the surface of the hydrophobic reversed particle is weaker than the protein, the length of the column has a greater influence on the separation of the polypeptide and the protein hydrolysate.
As shown in Figure 12, the resolution of peptides separated by long columns is usually higher than that of short columns.
Peptide separation is recommended for columns of 15 or 25 cm in length .
Figure 11. Adsorption and desorption of protein near the top of the column. After desorption, the protein hardly interacts with the hydrophobic phase, so increasing the length of the column does not increase the resolution of the protein, but increases the resolution with small molecules.

Figure 12. In contrast to proteins, peptides usually have higher resolution on long columns.
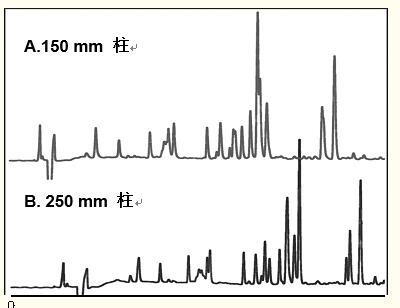
Column: C18 small hole, 4.6 x 150 or 250 mm
Eluent: Gradient: 0 - 70% acetonitrile, 60 min elution
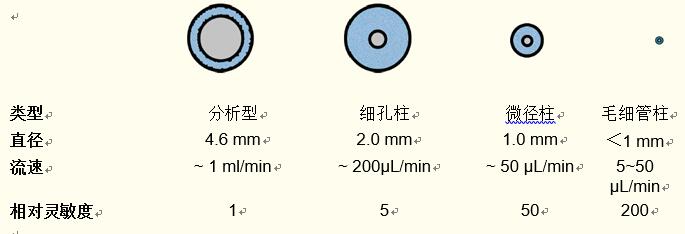
Column inner diameter . The analytical HPLC column has a standard internal diameter of 4.6 mm. The optimum flow rate for this type of column is ~1 ml/min.
Columns with smaller pore sizes are available on the market and are used to meet specific requirements and objectives.
The flow rate of the pore column (~2 mm inner diameter) is ~ 200 μl/min, so less solvent is used than the 4.6 mm inner diameter analytical column.
At the same time, the sensitivity of the pore column is about five times that of the standard analytical column.
This is because the amount of solvent flowing through the detector per minute is less, resulting in a higher peak concentration of protein or polypeptide.
Concentration detectors such as UV detectors and electrospray mass spectrometers are more sensitive to small pore columns.
The flow rate of the micro-diameter column is ~50 μl/min, so the sensitivity of the micro-diameter column is higher, about 50 times that of the analytical column.
The capillary column has a flow rate of 1 to 50 μl/min and has a higher relative sensitivity, which is about 200 times the sensitivity of the analytical column.
However, due to the flow rate used and the larger dead volume, special instruments are required for the micro-diameter columns and capillary columns.
Care must be taken when using micro-diameter columns.
The characteristics of the column are summarized in Figure 13 and the appendix.
Figure 13. Characteristics of different internal diameter columns
Pain Relief Patch for Breast
[Name] Medical Cold Patch
[Package Dimension] 10 round pieces
The pain relief patch is composed of three layers, namely, backing lining, middle gel and protective film. It is free from pharmacological, immunological or metabolic ingredients.
[Scope of Application] For cold physiotherapy, closed soft tissue only.
[Indications]
The patches give fast acting pain relief for breast hyperplasia, breast fibroids, mastitis, breast agglomera tion, swollen pain.
[How To Use a Patch]
Please follow the Schematic Diagram. One piece, one time.
The curing effect of each piece can last for 6-8 hours.
[Attention]
Do not apply the patch on the problematic skin, such as wounds, eczema, dermatitis,or in the eyes. People allergic to herbs and the pregnant are advised not to use the medication. If swelling or irritation occurs, please stop using and if any of these effects persist or worsen.notify your doctor or pharmacist promptly. Children using the patch must be supervised by adults.
[Storage Conditions]
Store below 30c in a dry place away from heat and direct sunlight.
Pain Relief Patch For Breast,Pain Relief Plaster For Breast,Relief For Breast Pain,Pad Relief Patch For Breast
Shandong XiJieYiTong International Trade Co.,Ltd. , https://www.xjplaster.com