Intermediate phase of clinical trial of lymphoma new drug is positive
March 15, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];German biopharmaceutical company MorphoSys recently released updated data on the ongoing single-group phase 2 clinical trial L-MIND. L-MIND test The efficacy and safety of the MOR208 antibody plus lenalidomide combination was studied in patients with relapsed refractory diffuse large B-cell lymphoma (DLBCL). MOR208 is an Fc-engineered monoclonal antibody directed against CD19 and is currently undergoing clinical development.

DLBCL is the most common malignant lymphoma in the world, accounting for approximately 30% of all non-Hodgkin's lymphoma. 30% to 40% of patients with DLBCL have no remission or relapse after first-line treatment. Patients who are unable to receive first-line therapy and are not suitable for high-dose chemotherapy (HDC) and autologous stem cell transplantation (ASCT) have a poor prognosis and patients are in desperate need of more treatment options.
CD19 is widely expressed in different types of B cell malignant cells, including DLBCL and chronic lymphocytic leukemia (CLL). According to related reports, CD19 can enhance B cell receptor (BCR) signaling, which is very important for the survival of B cells, so CD19 becomes a potential target for B cell malignancies.
The L-MIND study included patients with relapsed refractory DLBCL who did not receive HDC and ASCT and had received up to three prior treatments, at least one of which included anti-CD20 targeted therapy (eg rituximab) ). In November 2017, the trial completed the recruitment of 81 patients. The updated interim data for today's report included all 81 patients enrolled in the L-MIND trial, 68 of whom were evaluated by the investigator at the time of data cut-off. The median age of the enrolled patients was 72 years and received a median of two prior treatments.
The published data showed that of the 8.3 months of median observation, 33 of the 68 patients had remission, with an overall response rate (ORR) of 49% and 31% of patients with complete remission (CR). The initial progression-free survival rate (PFS) at 12 months was 50.4% (95% CI: 40-67%) and the initial median PFS was not met. Twenty-nine of the 33 patients (88%) were ongoing at the time of data cut-off. The median time to remission was 1.8 months and the median time to complete remission was 3.6 months. No unexpected toxicity was observed in the treatment combination and there was no report of an MOR208 infusion related response (IRR).
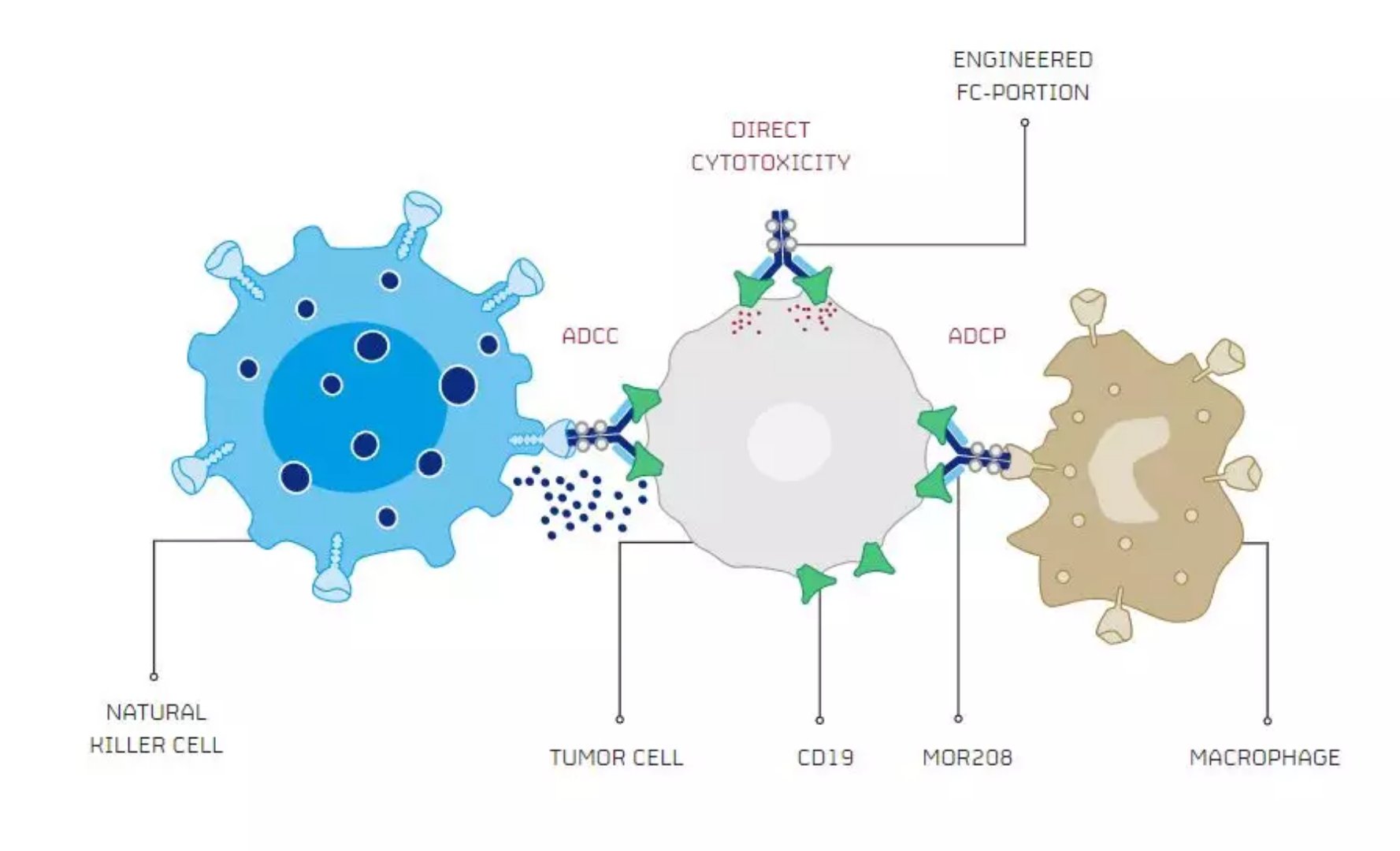
â–²The mechanism of action of MOR208 (Source: MorphoSys official website)
Dr. Malte Peters, Chief Development Officer of MorphoSys commented: "We are very excited about the data and the FDA's discussion of the market development of MOR208 under the breakthrough therapy, including accelerating regulatory submissions and approving the possibility of MOR208 based on the L-MIND study. Sexuality. We look forward to more mature data analysis from the L-MIND trial and interaction with the FDA."
"For patients with relapsed refractory DLBCL, there is a very high unmet medical need, and after initial treatment failure, they are not suitable for high-dose chemotherapy and autologous stem cell transplantation," Dr. Simon Moroney, CEO of MorphoSys said: " We are very encouraged by the latest clinical data from the ongoing L-MIND trial, which supports our program to develop MOR208 in combination with lenalidomide as a non-chemotherapy option for this patient population."
We expect this combination to bring relief to more patients with malignant blood cancer.
Reference materials:
[1] Tracking a successful DLBCL assault in Phase II, MorphoSys sets out to seize quick OK for MOR208
[2] MorphoSys Reports Updated Data from L-MIND Study of MOR208 plus Lenalidomide in Aggressive Lymphoma (r/r DLBCL)
[3] WuXi PharmaTech - treatment of lymphoma MOR208 was approved by FDA breakthrough therapy
Digital Pathology Slide Scanner
DIAN Biotech offers small-to-large routine pathology laboratories high-quality and high-speed digital scanning solution, from low-to-high throughput applications.
The digital pathology slide scanners are designed to meet diagnostic requirements, and guarantee constant color fidelity and high resolution histological and cytological samples, so that the image can be safely used to create a diagnosis.
Powered by unique scanner software, GENEDIAN digital pathology slide scanners can achieve one-touch, fast and high-quality slide scanning to meet the needs of routine pathology laboratories.
LBC, AI-assisted Diagnosis, Digital Pathology Slide Scanner
Hangzhou DIAN Biotechnology Co., Ltd. , https://www.dianbiotech.com