On March 7th, CMDE official website also announced the results of medical device priority approval application and medical device special approval application results: among them, 1 product was approved in priority, and 11 products were specially approved.

Medical device priority approval application review result announcement (No. 2, 2017)
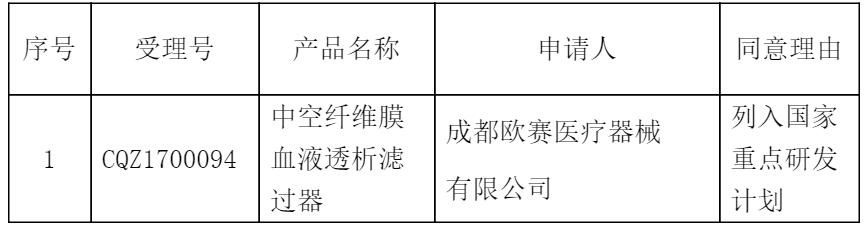
Publicity time: March 7, 2017 to March 13, 2017
During the publicity period, if any unit or individual has any objection, it may fill out the medical device priority approval project objection form and submit it in writing to the General Business Department of our center.
Announcement of the results of the examination of the application for the examination of the special medical equipment (No. 2, 2017)
1. Product Name: Drug-eluting peripheral vascular stent system
Applicant: Zhejiang Guichuang Medical Devices Co., Ltd.
2. Product Name: Hydrogen Oxygen Atomizer
Applicant: Shanghai Haomei Medical Technology Co., Ltd.
3. Product Name: Human SDC2 gene methylation detection kit (fluorescence PCR method)
Applicant: Guangzhou Kang Liming Biotechnology Co., Ltd.
4. Product Name: Neurosurgical Robot Navigation and Positioning System
Applicant: Huake Precision (Beijing) Medical Technology Co., Ltd.
5. Product Name: GNAS Gene Mutation Detection Kit
Applicant: Tianjin Jing Naite Gene Biotechnology Co., Ltd.
6. Product Name: Human EGFR gene mutation detection kit (multiplex fluorescent PCR method)
Applicant: Xiamen Aide Biomedical Technology Co., Ltd.
7. Product Name: Human cancer multi-gene mutation combined detection kit (reversible end termination sequencing method)
Applicant: Xiamen Aide Biomedical Technology Co., Ltd.
8. Product Name: Paclitaxel-eluting PTCA balloon dilatation catheter
Applicant: Zhejiang Batai Medical Technology Co., Ltd.
Ip Video Intercom,Ip Intercom System,Ip Video Intercom System,Ip Door Entry System
Zhuhai Mingke Electronics Technology Co., Ltd , https://www.mingke-tech.com