
Product Name: Tyvek wet heat sterilization bag
Brand: AC Sterile
Material Description: Unique Tyvek 1073B+ polyethylene film
Tyvek®'s unique structure (continuous and strong fibers) produces curved channels that achieve excellent microbial barrier properties and good strength properties. Made of high-density polyethylene (HDPE), it combines all the best features of paper, film and fiber. This unique balance of properties is unmatched in any other material, making Tyvek® lightweight, tough, breathable, water resistant, chemically resistant and resistant to puncture, tear and abrasion. In addition, Tyvek® lint has a low abrasion rate, smoothness and opacity.
Working principle: steam or sterilizing agent can enter and exit from the paper surface, bacteria can not pass, and sterilized in the breathing bag after sterilization
Product parameters:
| Mark color | Pink to black |
| Positive material | TYVEK 1073B |
| Fitting surface material | 90μm HDPE film |
| Pull value | Average ≥15N/15mm |
| particle | ≥5μ, ≤100pcs/ml ≥10μ, ≤20pcs/ml ≥25μ, ≤2pcs/ml |
| Validity period | Three years from the date of manufacture |
Product specifications:
Common product specifications can also be customized according to your needs.
| Product number | size | Packing specification |
| P103.11.7301 | 200mm x 300mm | 100 pieces / pack, 1000 pieces / box |
| P103.11.7305 | 400mm x 500mm | 100 pieces / bag, 500 pieces / box |
| P103.11.7307 | 500mm x 650mm | 100 pieces / bag, 200 pieces / box |
| P103.11.7310 | 800mm x 800mm | 50 pieces / bag, 100 pieces / box |
Scope of application: packaging of high pressure steam (121 ° C, 30 minutes), ethylene oxide, equipment for irradiation sterilization, rubber stoppers and clean clothes
Features:
1. Made of DuPont Tyvek (Tyvek) and HDPE film, good breathability and high antibacterial
2. The steam sterilization indicator mark is printed on the Tyvek surface material, and the color is used to identify whether the product has undergone a sterilization process.
3, a variety of specifications, can also provide customized size specifications according to requirements
4, in line with biocompatibility, insoluble particles, endotoxin and other standards
5, HDPE film is a transparent synthetic film, strong and durable, can quickly and clearly identify memory items
Product advantages (compared with medical wrapping paper):
1. Excellent barrier against microbial infiltration
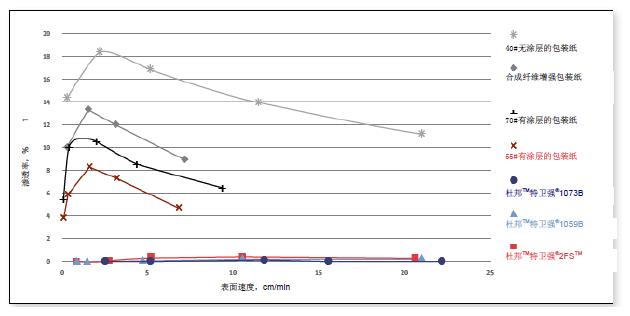 Particle Permeability of Porous Sterile Barrier Materials ( ASTM F2638 )
Particle Permeability of Porous Sterile Barrier Materials ( ASTM F2638 ) 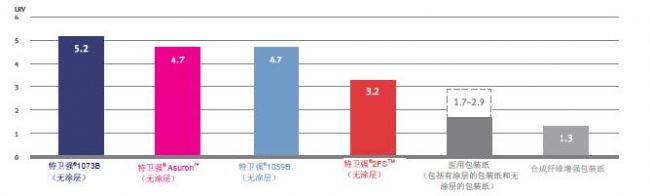
Microbial barrier test for sterile barrier materials ( ASTM F1608 )
The ability of the porous sterile barrier material to prevent bacterial spore penetration is measured in accordance with ASTM F1608, "Microbial Fractionation Standard Test Method for Porous Packaging Materials (Open Chamber Method)". Control sample that is completely impermeable (microbial permeability is zero). Under the challenge of one million or 106 colonies (cfu), the LRV value is 6, which is the base 10 logarithm of 106 colonies (CFU). is 6. If a sample facing the same challenge as the control sample allowed 10 colony forming units (cfu) (log10 = 1) to infiltrate, its log reduction value (LRV) was 5 (6-1 = 5). Therefore, the higher the log reduction value (LRV), the stronger the ability of the packaging material to resist microorganisms. 2, excellent tear strength and puncture resistance
DuPontTM Tyvek® continuous and strong fibers protect the packaging from product breakouts and penetration of external objects during rough handling. Compared to medical wrappers, Tyvek® has better puncture resistance and tear strength, which means that Tyvek® is not easily pierced, and if the package is cut, the tear does not easily spread.
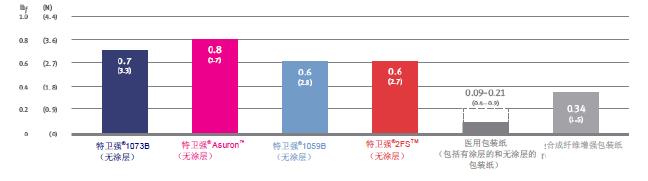
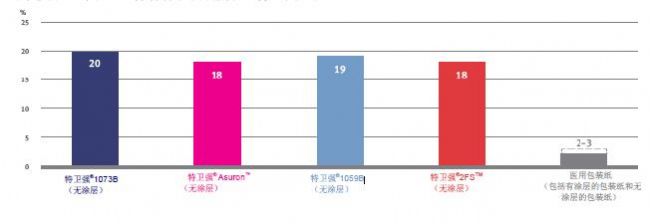
DuPont TM Tyvek ® products and medical packaging Elmendorf tear strength (MD) properties (ASTM D1424 and EN 21974)
This test measures the force required to expand the initial tear from the slit or gap. MD stands for the longitudinal direction, and the higher the value, the less likely the material is to be torn by force. 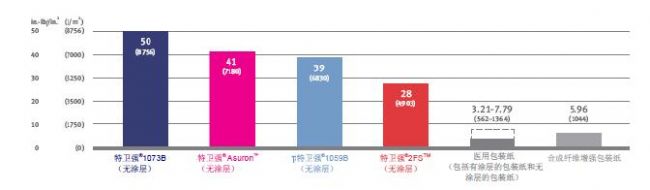
Spencer puncture DuPont TM Tyvek ® and medical packaging of products (ASTM D3420, procedure B)
The test measures the impact resistance of plastic films and packaging materials, and the test conditions are very close to the strain rate that these materials face when used in the health care industry. These results were obtained with a modified Spencer puncture tester featuring a hemispherical probe tip with a diameter of 9/16 inch (14.3 mm) and a 6400 gram pendulum for piercing Tyvek® and other toughness The material is essential. The results obtained with different test equipment are not comparable. 3, superior resistance to cracking
Tyvek® is an extremely flexible packaging material that is not as easily broken or torn as medical wrappers (Figure 7). The flexibility and inherent strength of Tyvek® ensure smooth operation of the form-fill-seal packaging line without significant downtime due to material failure.
 Elongation DuPont TM Tyvek ® products and medical packaging (MD) of characteristics (ASTM D5035 and EN ISO 1924-2,
Elongation DuPont TM Tyvek ® products and medical packaging (MD) of characteristics (ASTM D5035 and EN ISO 1924-2, The sample length is 5 inches [13 cm] and the elongation rate ( ROE ) is 2 inches / minute [5 cm / min]
4, excellent water resistance and moisture permeability
DuPontTM Tyvek® is highly resistant to water permeation. In fact, water that comes into contact with Tyvek® does not “wet†the Tyvek® surface, which means that the water does not diffuse but remains as water droplets on the surface. Unlike paper, Tyvek® products are hydrophobic and do not absorb water.
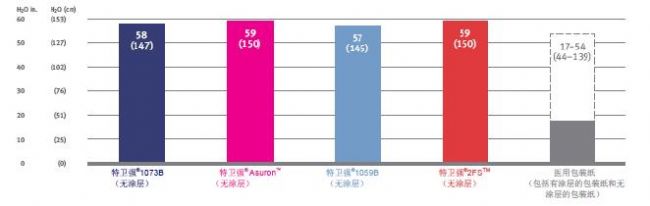
Hydrostatic characteristic DuPont TM Tyvek ® and medical packaging of products (AATCC TM 127 and EN 20811, pressure was
60cm H 2 O/min )
Hydrostatic pressure is an important indicator of the pressure required to pass three drops of water through a substrate. The higher the value, the stronger the water resistance of the package. 60cm H 2 O/min )
5. Adaptability to sterilization methods
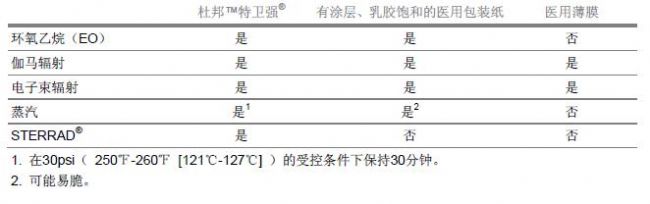
Unlike medical wrappers and films, DuPontTM Tyvek® is suitable for all the most common sterilization methods including: ethylene oxide (EO), gamma rays, electron beams, steam (under controlled conditions) and low temperature oxidation. Bacterial methods (eg STERRAD® Sterilization System). This is because Tyvek® is made of high-density polyethylene, which is extremely stable when exposed to sterile gases and high-energy sterilization methods. In addition, Tyvek® is specifically designed to allow rapid penetration and resolution of sterilizing gases and vapors. Regardless of the sterilization method used, Tyvek® maintains superior protection against microbial barriers and strength.
Table - Material Adaptability with Various Sterilization Methods
 6. Tyvek performance after aging
6. Tyvek performance after aging Tyvek® provides long-term aseptic maintenance to sterilized and packaged medical devices. The sterility of Tyvek® during storage of medical devices has been confirmed by aging studies and all medical devices on the market with a five-year lifespan.
Material-based research and results from a full-package shelf study show that:
• Tyvek® inhibits bacterial spores even under the toughest conditions.
• Bacteriological studies clearly demonstrate the remarkable effect of Tyvek® as a bacterial barrier, even after repeated challenges.
• Tyvek® remains sterile even after exposure to microbial contamination for five years.
Tyvek® retains its physical properties over time, maintaining package integrity.
User guides:
According to the "Guidelines for the Implementation of Sterile Preparations", for sterilized articles, enterprises should establish a sterilization management process to prevent secondary pollution after transfer and storage.
1. Wet-heat-sterilized cleansing companies should establish appropriate sterile dressing management procedures and avoid secondary contamination during storage prior to sterilization. For example, when using sterilized clothing for sterilization by damp heat sterilization, it is conceivable to use a breathable breathing bag for wrapping, so that the clothes can be sterilized for a longer period of validity (for example, one month), which is beneficial for the enterprise to fully utilize the sterilizer. At the same time, secondary pollution of the sterile clothing during storage can be avoided.
2. The transfer after the rubber stopper sterilization is suitable for the sterilization storage transfer of the halogenated butyl rubber plug to be washed, the halogenated butyl rubber plug to be rinsed, and the halogenated butyl rubber plug which only needs to be sterilized.
3. Transfer and preservation of equipment accessories related to sterilization and sterilization filtration process after autoclaving
 Precautions:
Precautions: 1. Check the sealing of the breathing bag before use.
2, according to the size of the sterilized items, choose the appropriate size of the breathing bag, should maintain a loose space around, should not be too tight, otherwise there is a risk of explosion in the process of repeated vacuum.
3. The name, code, batch number and other information of the sterilized item should be marked or printed on the blank surface of the breathing bag for identification and traceability.
Quality verification standards:
1. Appearance: The surface should be smooth and uniform in color, and there should be no perforation, foreign matter, odor, adhesion, heat sealing parts should be flat, no virtual seal.
2, insoluble particles: made into a sterilization bag with an internal surface area of ​​360cm2, add 250ml of particulate water for inspection, according to the method of infusion bottle and infusion bag in the packaging material insoluble particle test method (YBB00272004), particle diameter ≧ 5, 10, The number of particles of 25um must not exceed 100, 20, 2 / ml respectively.
3, bacterial endotoxin: made into a table with a 360 cm2 sterilization bag, add 250 ml of pyrogen-free water, after sealing the bag, placed in a (70 ° C ± 2 ° C) water bath for 2 hours, put the cold as a test solution, according to The bacterial bag endotoxin test method (People's Republic of China Pharmacopoeia 2015 edition) method gel method shall not exceed 0.25 EU/ml.
21" Sanitary Sleeve For Embryo Transfer
21 Inches Sanitary Sleeve,Disposable Sanitary Chemise,Embryo Transfer Cover,Et Sheath Cover
Jinan Mucho Commercial Inc. , https://www.muchovet.com