a "line" type of artificial islet
January 10, 2018 Source: New Vision of health: meet future
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];There are more than 1 million people with type 1 diabetes in the United States, and daily insulin injections are actually a matter of life and death. Although it cannot be cured, the research team at Cornell University has developed a device that can revolutionize the management of this disease.
For type 1 diabetes, insulin-producing pancreatic cell clusters (islets) are destroyed by the body's immune system. The research team led by Minglin Ma, an assistant professor in the Department of Biological and Environmental Engineering at the Faculty of Agriculture and Life Sciences, has devised a clever way to transplant hundreds to thousands of islet cells into patients. They are protected in a thin layer of hydrogel and, more importantly, this layer is adhered to a polymer line, which allows for easy removal and replacement when they can no longer be used. This article was published in the December 25th issue of PNAS entitled "Designing a Retrievable and Scalable Cell Encapsulation Device for Potential Treatment of Type 1 Diabetes" by co-authors Duo An and Alan Chiu.
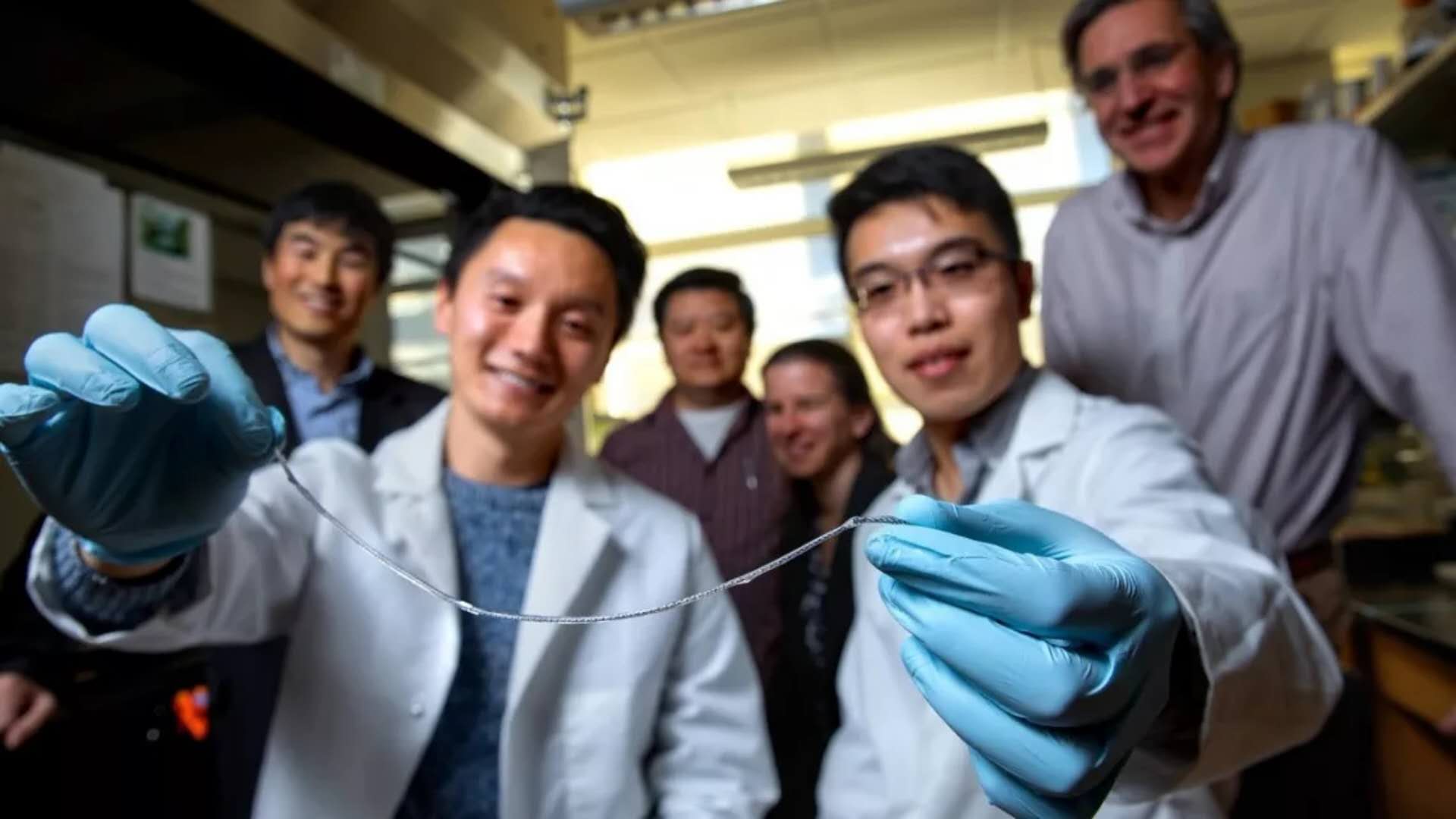
The research team presented the TRAFFIC sample. Image source: Cornell University
Stem cell transplantation, islet cells that produce insulin are another option for insulin therapy, but this requires long-term use of drugs to suppress the immune system. One way to avoid the immune system is to coat the islet cells with a thin layer of hydrogel, one hundred micrometers in diameter, to protect the islet cells. However, these small "capsules" are difficult to remove from the body because they are not connected to each other and are in the thousands. The ability to remove these implanted cells is critical because the stem cell-derived islet cells used have the potential to form tumors. “When they fail or die, they need to be taken out of the body,†Ma said. “You don’t want to put things out of your body. It’s not a problem with our methods.â€
Inspired by the water droplets on the spider web, Ma and his team first tried to connect the "capsules" containing islet cells through a single line, but they realized that it would be better to have the hydrogel evenly on the periphery.
This linear device is an ionized calcium release, nanoporous polymer wire. The device is first wound into a helical structure with two sterile nylon sutures and then folded to facilitate subsequent nanopore structure coating. Placed on this line is a thin layer of islet cells encapsulated in alginate hydrogel attached to a spiral nanopore line similar to dewdrops stuck to spider silk. Alginate is a seaweed extract that is commonly used to encapsulate cell transplants.
According to Ma, this device is called TRAFFIC (thread-enhanced alginate fiber for islet encapsulation) and is inspired by spider webs. The hydrogel evenly covers the surface of the wire, so this line is better. He said: "There is no gap between the capsules. With spider silk, there are still gaps between the water drops. In our case, the gaps cause scar tissue and similar scars." And because the lines are twisted and porous, So the hydrogel will not fall off as easily as on a single smooth piece of material.
Ma said that the large surface area of ​​TRAFFIC promotes a better mass transfer and delivery is good because all islet cells are near the surface. The current life cycle of this line is estimated to be 6 to 24 months, although more testing is necessary.
In mice, blood glucose was converted to normal levels two weeks after implantation of one inch of TRAFFIC, and remained normal until at least three months prior to the end of the experiment. Recyclability was tested in multiple dogs and a 10-inch sample was successfully implanted and removed by laparoscopy. Dr. James Flanders from the Veterinary College, who performed surgery in dogs, said that there was no or minimal adhesion between the device and the surrounding tissue between the different dogs and the device being tested. TRAFFIC received patent protection with the help of the Cornell Technology Licensing Center.
Flanders said: "When Minglin first told me about this, I thought it was great. Although there are other similar devices, this seems to have a lot of hope. It is the least reactive and can protect islet cells. They can make them feel glucose, they don't stick to anything, and they're easy to remove. For me, it sounds like a win-win situation."
We look forward to this special line-type device that will help more patients with type 1 diabetes in the future.
Reference materials:
[1] Removable implant may control type 1diabetes
Insulin Syringes Needle,Disable Syringe,Monoject Syringe,10 Ml Syringe
FOSHAN PHARMA CO., LTD. , https://www.forepharm.com