According to the data of the intranet, in the first half of 2019, 16 pharmaceutical companies in the United States seized 41 ANDA application numbers, involving 39 varieties (by drug name + company name), compared with the same period last year. Increased. East Sunshine and Haizheng Pharmaceuticals tied for the first place with six ANDAs, and the domestic face of domestic pharmaceutical companies ushered in a new face – China and the United States East China. 17 of the 39 varieties have been listed domestically or applied for listing/clinical in China, and 22 varieties are still being reported to the country.
Table 1: Chinese pharmaceutical companies obtained FDA-approved ANDA in the first half of 2019
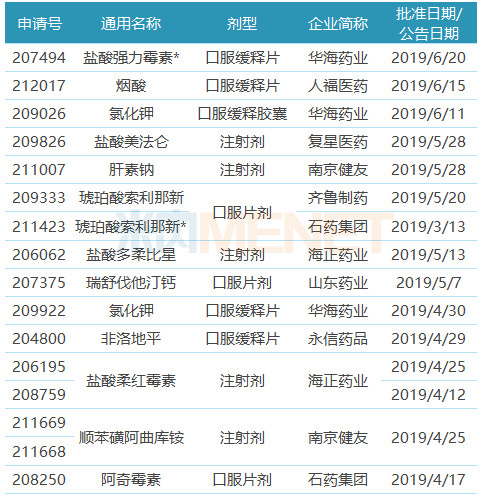
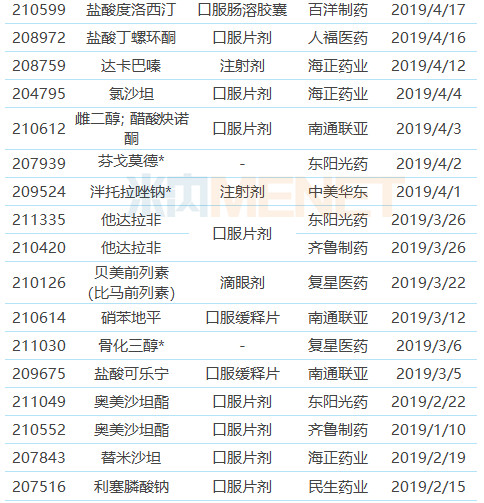
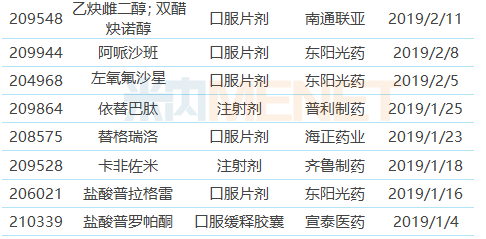
Note: With * is temporarily approved, the same below
(Source: Minenet FDA Drug Catalog)
According to the FDA drug catalogue of the intranet, in the first half of 2019, a total of 16 Chinese pharmaceutical companies obtained FDA-approved ANDA, a total of 41 application numbers, involving 39 varieties (by drug name + company name), compared with the same period last year. increase. The main reasons for the increase are:
On the one hand, the US policy encourages the listing of generic drugs. For the first ANDA generic drug that has challenged the original R&D and successfully listed, it will receive a 180-day market exclusivity.
On the other hand, the domestic generic drug industry is undergoing major changes. In recent years, the government departments have achieved the goal of “quality improvement†through the evaluation of the consistency of generic drugs, and promoted the “price reduction†of drugs through the “4+7†national procurement and 77 pharmaceutical enterprise accounting information quality inspections. In addition, Indian pharmaceutical companies have long been planning to seize the market in China, and the recent progress has accelerated. The entry of Indian medicine into the Chinese market may be one of the driving forces to stimulate the upgrading of the local generics industry.
On the other hand, the dividends of China and the United States are stimulated. The domestic pharmaceutical manufacturers have already approved the listing of generic drugs in the EU, the United States and Japan. Based on the relevant information on foreign registration, the drugs are listed in accordance with the new registration of chemical drugs. After the approval of the listing, it is deemed to pass the consistency evaluation; the drugs produced in China using the same production line and approved for listing in the EU, the US and Japan are deemed to have passed the consistency evaluation.
Figure 1: Approval of domestic pharmaceutical companies ANDA in the first half of 2019 (unit: one)
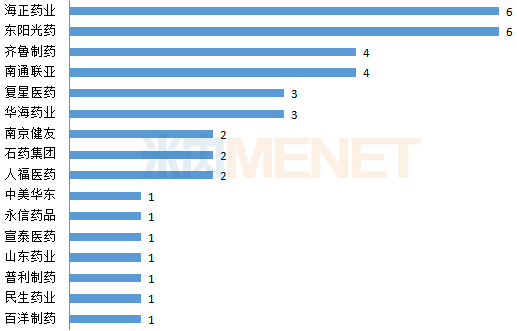
Note: based on the number of varieties (drug name + company name)
(Source: Minenet FDA Drug Catalog)
To date, 39 Chinese pharmaceutical companies (by parent company) have received FDA-approved ANDA. In the first half of 2019, the domestication of domestic pharmaceutical companies ushered in a new face - Hangzhou, China and the United States East. In the past, Huahai Pharmaceutical was affected by the incident of Shatan's API. In the first half of 2019, three ANDA were harvested. East Sunshine and Hisun Pharmaceuticals were eye-catching and captured 6 ANDA.
7 varieties are listed in China, and 4 have passed the consistency evaluation.
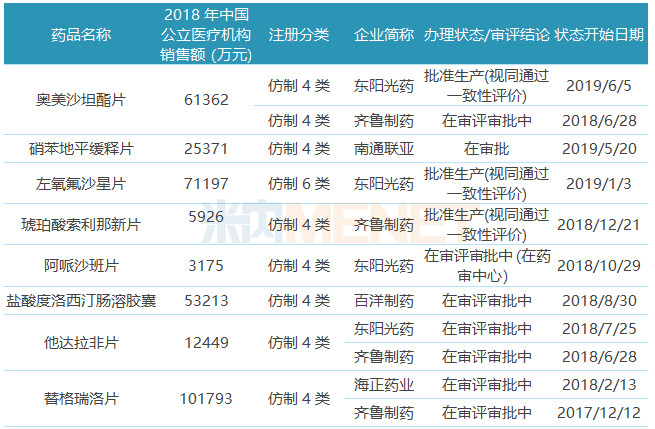
Table 2: Varieties that have been listed in China or declared for listing/clinical
(Source: Minenet database)
17 of the 39 ANDA varieties have been listed domestically or submitted for listing/clinical applications in China, pantoprazole sodium from Sino-US Huadong Pharmaceutical, daunorubicin and doxorubicin from Haizheng Pharmaceutical, and Shijiazhuang Group Azithromycin has been approved for production in China long ago.
Pantoprazole sodium for injection is the first approved ANDA of Huadong Medicine . At present, there are 68 manufacturers with approvals for this product in the domestic market. Sino-US Huadong Pharmaceutical, Yangzijiang Pharmaceutical, Osei Kang Pharmaceutical, and Nanjing Zhengda Tianqing have submitted The supplementary application for the consistency evaluation of the product is in the state of “in the process of review and approvalâ€; the azithromycin tablets of Shijiazhuang Group submitted the application according to the consistency evaluation supplementary application and passed the consistency evaluation. At present, 10 varieties of the company have passed or passed the same Consistency evaluation, in addition to azithromycin tablets, tramadol hydrochloride tablets, clopidogrel hydrogen sulfate tablets, metformin hydrochloride tablets have also obtained ANDA in the United States.
Fengomod and prasugrel of Dongyang Pharmaceutical are clinically reported in the new drug category 3.1, and these two varieties are still in a blank state in the domestic market. The competition for the first prasugre competition in China was fierce. Including Dongyang Sunshine, a total of 17 domestic pharmaceutical companies and one multinational pharmaceutical company's prasugrel hydrochloride tablets were approved for clinical trials.
Nine varieties were listed on the generic 4/6 category. The olmesartan ester tablets of Dongyang Pharmaceutical and the new succinate succinate tablets from Qilu Pharmaceutical were approved and passed the consistency evaluation. Qilu Pharmaceutical is amber. The new product of sourina was first evaluated by the pharmaceutical company; the levofloxacin tablets of Dongyang Pharmaceutical were listed on the generic 6 category, but because they are collinear products at home and abroad, they are deemed to have passed the consistency evaluation after being approved. This product is the first in China to comment. Nantong Lianya's nifedipine sustained-release tablets and Baiyang Pharmaceutical's duloxetine hydrochloride enteric-coated capsules are still under review and approval, and it is expected that the first one will pass the consistency evaluation.
The double-dividend bonus at home and abroad is obvious, and 22 varieties are expected to be reported to China.
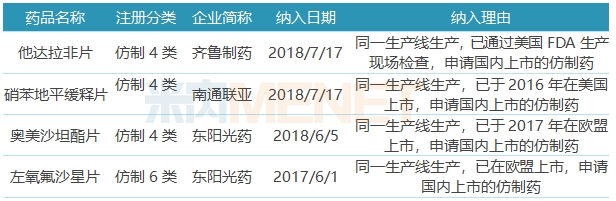
Table 3: Inclusion of priority review varieties
(Source: Mine database, CDE official website)
Four of the 17 ANDA varieties that have been listed domestically or submitted for listing/clinical applications in the country are included in the priority review, including the reason that “the same production line is produced, which has been listed in the EU or the US, and applied for domestically listed generic drugsâ€. Incorporating a priority review process helps accelerate time-to-market.
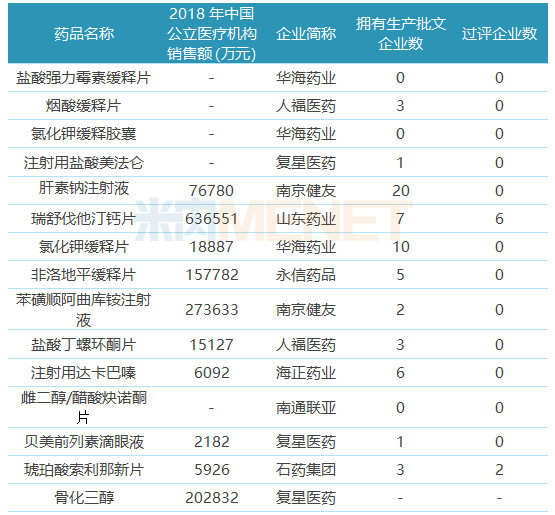
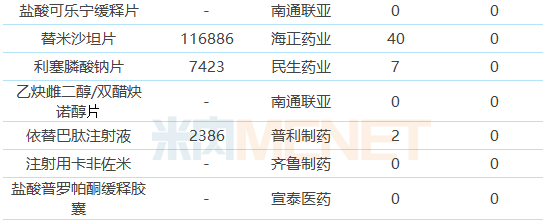
Table 4: ANDA Varieties Not Listed in the Country/Clinical
(Source: Minenet database)
Twenty-nine of the 39 ANDA varieties have not yet been applied for listing in the country or applied for clinical treatment. Among them, 7 varieties are still in the blank state in the domestic market. There are 2 varieties currently only the products of the original research manufacturers are listed and sold in China. Conducive to taking the lead in seizing the market.
Among the 22 domestic varieties to be transferred, only rosuvastatin calcium tablets and new succinate succinate tablets were approved by the company or passed the consistency evaluation. “Overseas transfer to the domestic market†has become a shortcut to pass the consistency evaluation in China. Typical representatives include Huahai Pharmaceutical and Dongyang Pharmaceutical. Huahai Pharmaceutical currently has 12 varieties reviewed, 10 of which have obtained American ANDA; There are 6 varieties of drugs reviewed, and 4 of them have obtained American ANDA.
The consistency of injection consistency evaluation progressed slowly. Among the currently evaluated varieties, only azithromycin for injection was submitted according to the consistency evaluation supplementary application and passed the consistency evaluation. The other injections were listed according to the new registration classification, and the approval was passed after approval. Evaluation. Six of the 22 domestic varieties to be converted are injections, and no enterprise has commented.
Source: Minenet database, CDE official website
Note: Data statistics as of July 1, if there are omissions, please correct me!
Organic Inulin Powder,Inulin Powder Dietary Fiber,Natural Dietary Fiber
Qingdao Bailong Huichuang Bio-tech Co., Ltd. , https://www.sdblcycn.com